Figure 7.
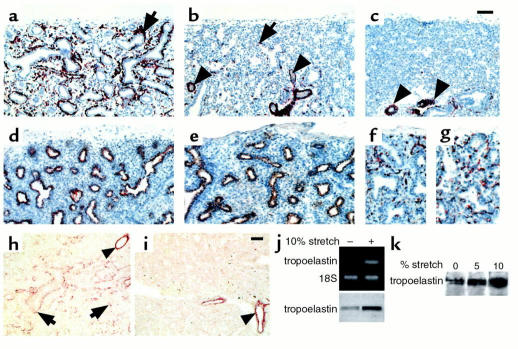
Immunohistochemistry showing paucity of visceral SM cells in human fetal hypoplastic lungs. Shown are histological sections from normal lung (a), hypoplastic lung caused by oligohydramnion (b), and hypoplastic lung caused by diaphragmatic hernia (c), all at 22 weeks of gestation, immunostained for SM α-actin. There is a significant decrease in bronchial and interstitial SM cells (arrows) in the hypoplastic lungs (b and c), particularly in those compressed by intrathoracic herniation of abdominal viscerae due to diaphragmatic hernia (c). The vascular musculature seems unaffected (arrowheads). In the same hypoplastic lung shown in b, the epithelial cells, immunostained for low–molecular-weight cytokeratins (e), and the endothelial cells, immunostained for PECAM-1 (g), show no changes compared with controls (d and f). Photos (h and i) demonstrate immunohistochemistry showing decrease in tropoelastin deposition in human hypoplastic lungs. (h) Histological sections from the normal lung at 20 weeks of gestation demonstrate tropoelastin deposition around bronchi and bronchioli and at scattered interstitial sites (arrows). (i) Histological sections from same age hypoplastic lung reveals essentially no tropoelastin deposition, with the exception of vascular SM that shows no changes in tropoelastin when compared with controls (arrowheads). Bar, 60 μm in a–e and h and i. Bar, 100 μm in f and g. (j) RT-PCR and immunoblot show stretch-induced upregulation of tropoelastin expression in mouse lung embryonic mesenchymal cells undergoing myogenic differentiation. (k) Immunoblot shows stretch-induced upregulation of tropoelastin synthesis in human lung embryonic mesenchymal cells undergoing myogenic differentiation. Results shown in j and k are representative of three experiments conducted in duplicate sample per treatment.