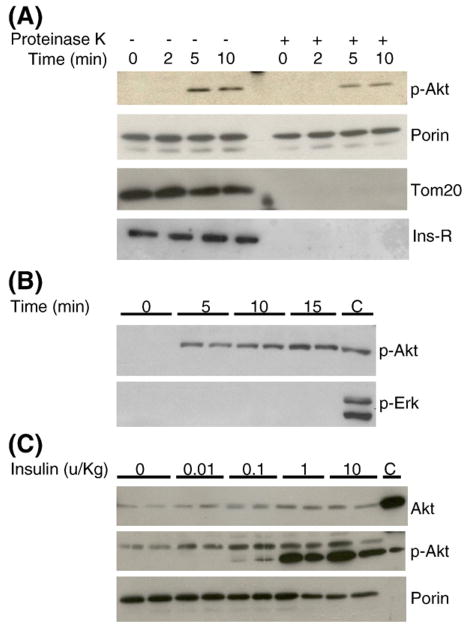
Fig. 5.
Insulin stimulates phosphorylation and translocation of Akt to mitochondria in cardiac muscle. (A) Insulin increased accumulation of p-Akt in myocardial mitochondria. Proteinase K digestion did not alter p-Akt translocation to mitochondria. Myocardial mitochondria preps were digested with proteinase K as described in the Research design and methods section to remove non-mitochondria proteins. TOM 20, a mitochondria protein, served as a control for proteinase K digestion. Immunoblotting with anti-insulin receptor β subunit antibodies showed that crude mitochondria preps were contaminated with insulin receptors (Ins-R) and were removed after proteinase K digestion. (B) Time-course of insulin stimulation on phospho-Akt translocation in primary cardiomyocytes. Cardiomyocytes were incubated with insulin (10−7 M) after overnight fasting. Equal protein amounts of proteinase K-treated mitochondria preparations were used for western blots. (C) Dose–response of insulin stimulation on phospho-Akt translocation in myocardium in vivo. Mice were overnight-fasted and injected with insulin, myocardial mitochondria were isolated and equal protein amounts of proteinase K-treated mitochondria preps were loaded to each lane. In the phospho-Akt blot, the lower band represents p-Akt and the upper band is a non-specific band. The last lane (C) represents total myocardial lysates from insulin-stimulated mice, as positive control. Immunoblot with anti-porin antibodies served as loading control.