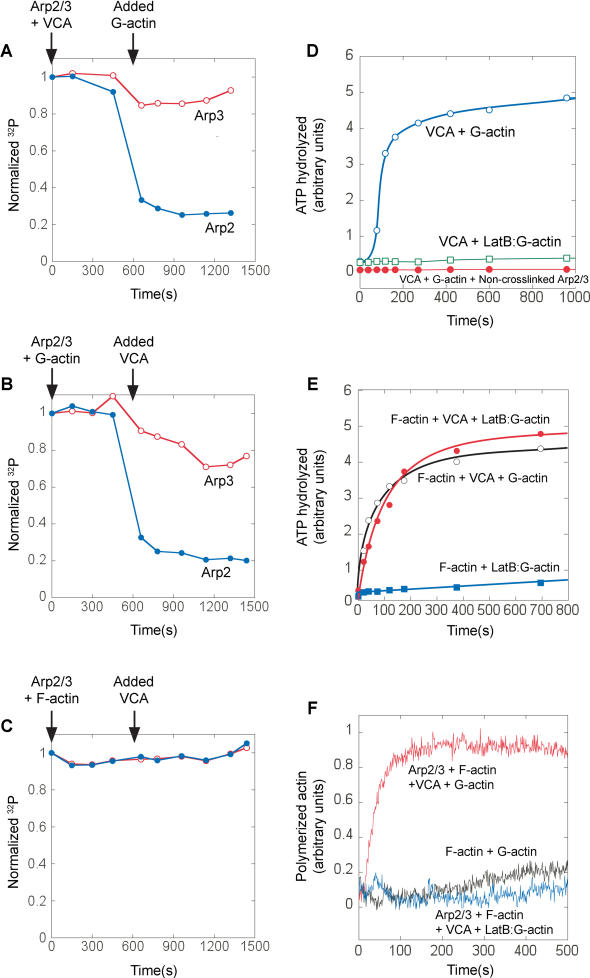
Figure 3. A Single Actin Monomer, in the Presence of Actin Filaments and VCA, Stimulates ATP Hydrolysis on Arp2, without Requiring Actin Polymerization.
(A–C) Remaining unhydrolyzed γ-32P-AzidoATP on Arp2 (closed circle) and Arp3 (open circle) was quantified to assay ATP hydrolysis (same conditions as Figure 1B–1D). γ-32P-AzidoATP-labeled Arp2/3 (20 nM) was mixed at indicated times with either 750 nM VCA then 2 μM G-actin (A), 2 μM G-actin then 750 nM VCA (B), or 2 μM F-actin then 750 nM VCA (C).
(D) Latrunculin B (open square) inhibits the ability of VCA plus monomeric actin (open circle) to stimulate ATP hydrolysis on the Arp2/3 complex in the absence of actin filaments. Also, 32P ATP hydrolysis signal requires covalent crosslinking to Arp2/3. Arp2/3 was mixed with 6 μM γ-32P-AzidoATP and exposed to UV either before (closed circle) or after (open circle) the addition of excess (2 mM) unlabeled ATP. Excess ATP added before the UV exposure prevents crosslinking and abolishes the ATP hydrolysis signal, indicating that all the 32P ATP hydrolysis signals measured are due to ATP hydrolysis on Arp2/3 and not from ATP hydrolysis on actin.
(E and F) In the presence of phalloidin-stabilized actin filaments, actin monomers are prevented from polymerizing by Latrunculin B, but still stimulate ATP hydrolysis on the Arp2/3 complex. 20 nM γ-32P-AzidoATP–labeled Arp2/3 was premixed with 1 μM phalloidin-stabilized actin filaments. The reaction was initiated by mixing with 750 nM N-WASP VCA, 1 μM G-actin and 4 μM Latrunculin B as indicated, cleaved γ-32P was assayed by phosphomolybdate extraction (E), and separately, actin polymerization was monitored by pyrene–actin fluorescence (F).