Abstract
Osteosarcoma is the most common malignant primary neoplasm of bone. For an optimal oncological outcome, surgical removal of tumor is an essential component of its multidisciplinary treatment. Limb salvage surgery has long been established as the standard of care for osteosarcoma. While limb-salvaging techniques have acceptable rates of disease control, amputation remains a valid procedure in selected cases. In current orthopedic oncology practice, the focus is on optimizing the balance between preservation of form and function of the limb and adequate oncological clearance at the same time. Improving the functional outcome and longevity of reconstructive procedures also remains a challenge.
Keywords: Current concepts, Surgical treatment, Osteosarcoma
1. Introduction
Osteosarcoma is the most common malignant neoplasm of bone.1 Over the past 3 decades, the prognosis for patients with osteosarcoma has changed dramatically. Though the development of effective chemotherapy agents has reduced the incidence of metastatic disease and mortality,2 surgical excision of the tumor remains a most essential component of osteosarcoma management. This article reviews the current status of surgical treatment of osteosarcoma, including the indications and advantages of limb salvage, and techniques of resection and reconstruction.
The surgical treatment of osteosarcoma has historically been amputation/disarticulation. As early as 1879, it was realized that the most ablative of surgeries will not result in cure of the vast majority of patients.3 It was not until 1970s that the role of chemotherapy in improving survival in osteosarcoma patients was established.4 While there has been improvement in survival from inclusion of chemotherapy on the one hand, it has been paralleled by a shift of surgical treatment from amputation toward limb salvage surgery. More than 90% of patients with osteosarcoma undergo limb salvage surgery at most specialized centers.5 This change has resulted from advancements in prosthetics, surgical techniques, anesthesia, imaging and pathology.
2. Amputation or limb salvage
It has been established beyond doubt that the survival of patients with osteosarcoma is not adversely affected by the choice of limb salvage as the surgical treatment as against amputation.6 This has led to a protocol of considering every patient for limb salvage surgery at all specialized centers. However, limb salvage should be considered in a patient only if the surgeon is reasonably confident that surgical excision of the tumor with wide margins is feasible, and that the expected function of the limb after limb salvage surgery will be better than ablative surgery in the form of amputation/disarticulation. Limb salvage surgery will usually be contraindicated if there is pancompartmental disease with fungation, gross infection, encasement of major neurovascular bundle, and displaced pathological fracture not healing on neoadjuvant chemotherapy.7 While many workers have suggested marginally higher rates of local recurrence following limb salvage surgery compared to a radical amputation/disarticulation, it has been observed that this is not translated into statistically significant disadvantage in survival.6,8 On functional and cost of treatment parameters, limb salvage surgery scores above amputation, as recent studies indicate.9 Surprisingly, however, the long term psychological outcome of patients with limb salvage surgery is reported to be the same as amputation.10
3. Biopsy
Biopsy is the first and a very important part of the overall management of osteosarcoma. It is vital, like all other malignancies, in the confirmation of diagnosis. A core needle biopsy carried out as an outpatient/day care procedure is usually performed in the majority of patients, though an incisional biopsy may be required in a small minority of patients. Usually the soft tissue component is biopsied and the biopsy tract is placed in line with the planned definitive surgical incision, so that it does not violate tissue planes and neurovascular structures, and can be excised during definitive surgery. The biopsy should best be performed by an experienced orthopedic oncologist, working as part of a team finally doing the surgery.11
4. Timing of surgery in multimodal management
For low grade osteosarcoma (whether juxtacortical or central) wide surgical excision needs to be done as the only treatment. For high grade osteosarcomas, however, surgery has to be combined with multiagent chemotherapy. Initially, chemotherapy was given as a neoadjuvant (prior to surgery) treatment, with the idea that micrometastases will be addressed earlier, and there will be time to order and manufacture customized prosthesis. The modern prostheses now available are modular and available off the shelf, and do not require the waiting period for manufacturing. Moreover, neoadjuvant chemotherapy has shown no improvement in survival in numerous studies.6 However, neoadjuvant chemotherapy is still usually followed, as it gives the patient and the surgeon time to discuss and plan the resection and reconstruction, facilitates surgery by way of better defined tissue planes and resolved tissue edema, and gives a chance for prognostication of the patient by postoperative histopathological inputs. Surgery is then followed by adjuvant therapy, which may or may not be tailor made according to a good or bad histopathological response.
5. Resection
In a classic study performed at the Istituto Ortopedico Rizzoli, the different relevant margins in the treatment of osteosarcoma were charachterised.12 Surgical margins are defined as intralesional, marginal, wide, and radical. An intralesional margin is created if the tumor is entered at any point during surgery. A marginal margin is created when the dissection extends into or through the reactive zone that surrounds the tumor. A wide margin is created when the reactive zone is not entered and the entire dissection is performed through healthy tissues. A radical margin is created when the entire bony or myofascial compartment or compartments containing the tumor is resected.
The principle of surgical resection of osteosarcoma (as for any sarcoma of bone) is resection with wide margins (removal of tumor with a cuff of normal tissue covering it all around). This usually means removal of 2 cm normal tissue or a good anatomical barrier (e.g fascial layer/articular cartilage)13 and osteotomy of bone 3–5 cm away from the level of involvement. There have also been recommendations of smaller margins on bone being acceptable for resection after effective neoadjuvant treatment.13 Joint sparing resections using the open physeal cartilage as margin are also oncologically sound, while saving the nearby joint at the same time. Some workers have advocated the use of computer navigation for accurate resection with safe margin based on imaging findings while preserving as much bone as feasible.14 Similarly, distraction of growth plate is also being done preoperatively to enable preservation of the physis while retaining good margins of excision.15 An intraoperative frozen section from the bone marrow should be sent for confirmation of negative margin at the osteotomy site. Ablative surgery in the form of amputation or disarticulation is indicated in cases where salvaging the limb is not feasible with resection of tumor with wide margins.
6. Reconstruction
Reconstruction of large segmental defects following resection is a challenging task. An ideal reconstruction should be durable, compensate for the loss of growth of the involved limb in skeletally immature patients, result in the function and appearance of the limb as close to normal as possible, be compatible with early rehabilitation, and be cost effective and readily available. Obviously, there is no single ideal method of reconstruction, and it has to be chosen keeping in mind the requirements of the patient.
6.1. Reconstruction using megaprosthesis
Reconstruction with megaprosthesis is a common mode of reconstruction as it has a predictable functional outcome, allows early rehabilitation, allows for intraoperative flexibility in the length of the reconstruction required and being non biological, is unaffected by adjuvant chemotherapy (Figs. 1 and 2). However, the main disadvantage of megaprosthesis is the vulnerability to wear and tear leading to loosening/breakage in the long term. Furthermore, the reattachment of tendons to the prosthesis is another factor compromising the functional outcome.16 Availability of expandable prosthesis has minimized the problem of limb length discrepancy in young children with significant remaining growth, as they can be lengthened non-invasively.17
Fig. 1.
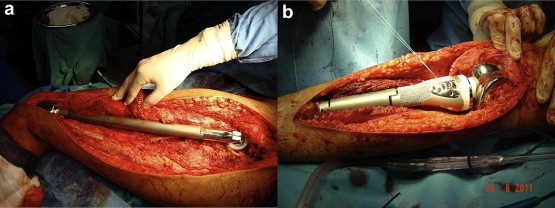
A total femur (a) and a proximal tibia (b) megaprosthesis in situ.
Fig. 2.
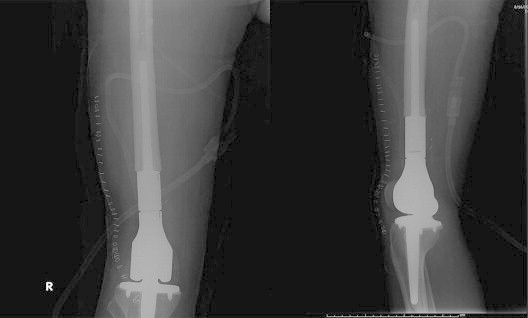
X-ray showing a distal femur megaprosthesis.
6.2. Biological methods of reconstruction
Biological reconstruction may be used for arthrodesis (Fig. 3), intercalary reconstruction (Fig. 4), or osteoarticular graft. They depend on bone healing for rehabilitation, which is subject to effects of adjuvant therapy and is associated with a long rehabilitation time. Osteoarticular allografts offer the advantage of good reattachment of tendons for optimal function, particularly at sites such as proximal tibia, proximal femur and proximal humerus. However, the availability of cadaveric grafts is limited, and the issues of infection, graft fracture, non union and osteoarthritis are reasons for concern.18,19 They can also be used as allograft – prosthesis composite, to avoid development of early osteoarthritis.20
Fig. 3.
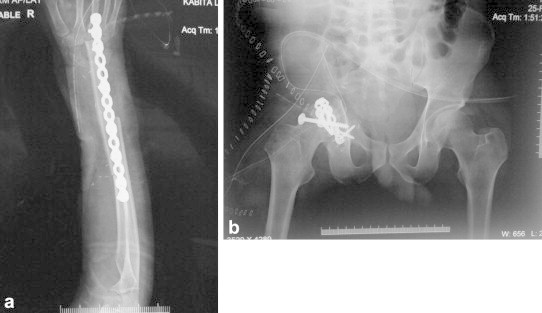
Arthrodesis of (a) wrist following distal radius resection and vascularized fibula graft (b) hip following internal hemipelvectomy.
Fig. 4.
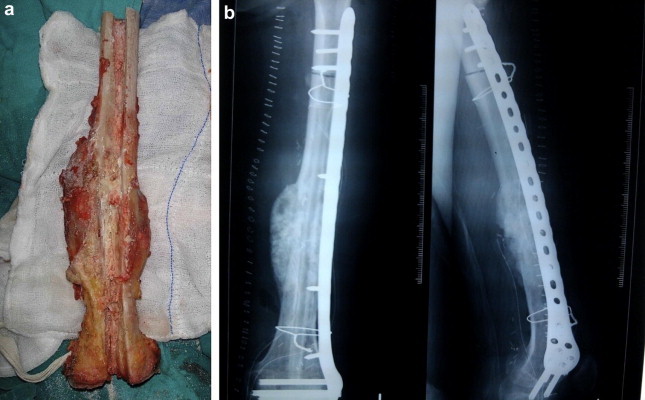
(a) Tumor excised and irradiated, prepared for implantation (b) Extracorporeally irradiated bone reimplanted (augmented with vascularized fibula graft).
In centers not having cadaveric bone bank access, resected tumor may be extracorporeally treated (radiotherapy/liquid nitrogen/pasteurization) and reimplanted to reconstruct the defect. This method offers a massive bone graft exactly matching the requirement of the defect created, and is highly cost effective (being the patient's own bone) (Fig. 4). However, the indications are limited and the postoperative histopathological input is suboptimal.21,22
Vascularized autografts are also used for intercalary defects, arthrodesis (Fig. 3), or as growing osteoarticular grafts. They unite more predictably and show earlier hypertrophy compared to nonvascular autografts.23 Vascular autografts are also very useful when combined with allograft/irradiated bone, providing vascularity to these massive grafts and making the outcome more predictable (Fig. 4).
7. Management of complications
7.1. Infection
Periprosthetic infections are a frequent (reported rates approximately 10%) complication of limb salvage surgery which is largely due to prolonged and repeated surgeries, as well as to the immunocompromised condition of these patients. Furthermore, the large exposure of tissues and extensive dissection across vascular distributions also contributes to the high risk of infection. The highest risk of infection has been observed after proximal tibia resection due to the poor soft tissue coverage and pelvic resection due to dead space and vicinity to pelvic viscera.24 Radiation therapy and expandable prosthesis are also reported to be risk factors for infection.25
Usually one or more attempts at debridement with antibiotic therapy (systemic and local antibiotic cement beads) are indicated as first line treatment of infection of tumor megaprosthesis, particularly in the early postoperative setting. If these measures don't work, implant removal and thorough debridement and lavage is indicated. Usually an antibiotic impregnated cement spacer is placed before a new implant is inserted as a two staged procedure. Amputation may be ultimately required in a fair proportion of these patients.25
7.2. Local recurrence
Local recurrence occurs in about 5% of patients undergoing limb salvage surgery for extremity and girdle osteosarcomas at specialized centers. The treatment of a local recurrence depends on the timing of recurrence, association with distant metastases, and resectability. Resectability is decided on the same criteria as a primary tumor, and local recurrence does not always warrant an amputation. A short disease free survival or an association with pulmonary metastases may warrant first or second line chemotherapy.26 While local recurrence of osteosarcoma usually carries a poor prognosis,27 there have also been reports of local recurrence not having a significant impact on overall survival.28
7.3. Implant failure
Mechanical failure is the commonest reason for failure of reconstruction with megaprosthesis.29 Long term series report a survival rate of tumor megaprosthesis at 10 years ranging from 50 to 90% approximately, the highest revision rates being with proximal tibia implants.30,31 There has been a lot of research into efforts to improve the longevity of tumor megaprosthesis, and improvements in prosthetic technology (rotating platform design, HA coated collar and stem, porous tantalum and compression osteointegration technology) hold promise in overcoming these limitations of this useful method of reconstruction.16
7.4. Non union
Non union/fracture of biological reconstruction (arthrodesis/intercalary reconstruction) using allograft/vascular autograft/nonvascular autograft may be seen in patients undergoing these procedures. The effect of adjuvant chemotherapy/radiotherapy, and use of nonvascular bone (e.g. allograft/extracorporeally treated bone) leads to a greater incidence of these complications.
8. Management of metastatic disease
Metastatic osteosarcoma generally carries a poor prognosis. The treatment has to be individualized to the patient, depending on the site (pulmonary or extrapulmonary), number and time of presentation. Pulmonary metastases, few in number, resectable and presenting late with a long doubling time carry a better prognosis and are good candidates for pulmonary metastectomy.32 There have even been reports of improvement in longevity of patients undergoing repeated pulmonary metastectomies.33 These considerations have led to a more aggressive approach to treatment of metastatic osteosarcoma in recent times.
9. Conclusion
Surgical management of patients with osteosarcoma is challenging. No difference in survival has been shown between amputations and adequately performed limb-salvaging procedures. Optimal tumor resection and a functional residual limb with increased survival of both the patient and the reconstruction are the goals of today's orthopedic oncology. Removal of tumor with adequate margins should be the primary consideration, whether the surgery is limb sparing or limb sacrificing. Reconstruction should be individualized to the needs of the patient keeping in mind the oncological, functional and social requirements.
Conflicts of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Biermann J.S., Adkins D., Benjamin R. Bone cancer. J Natl Compr Canc Netw. 2007;5:420–437. doi: 10.6004/jnccn.2007.0037. [DOI] [PubMed] [Google Scholar]
- 2.Lewis V.O. What's new in musculoskeletal oncology. J Bone Joint Surg Am. 2007;89:1399–1407. doi: 10.2106/JBJS.G.00075. [DOI] [PubMed] [Google Scholar]
- 3.Gross S.W. Sarcoma of the long bones, based on a study of one hundred and sixty five cases. Am J Med Sci. 1879;78:338. doi: 10.1097/01.blo.0000183422.05747.b8. [DOI] [PubMed] [Google Scholar]
- 4.Sweetnam R. Osteosarcoma. Br Med J. 1979 Sep 1;2(6189):536–537. doi: 10.1136/bmj.2.6189.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picci P. Osteosarcoma (osteogenic sarcoma. Orphanet J Rare Dis. 2007 Jan 23;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goorin A.M., Schwartzentruber D.J., Devidas M., Pediatric Oncology Group Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003 Apr 15;21(8):1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 7.Malawer M. Distal femoral resection with endoprosthetic reconstruction. In: Malawer M.M., Sugarbaker P.H., editors. Musculoskeletal Cancer Surgery Treatment of Sarcomas and Allied Diseases. Kluwer Academic Publishers; Dordrecht: 2001. pp. 457–482. [Google Scholar]
- 8.Rougraff B.T., Simon M.A., Kneisl J.S., Greenberg D.B., Mankin H.J. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994 May;76(5):649–656. doi: 10.2106/00004623-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Grimer R.J., Carter S.R., Pynsent P.B. The cost-effectiveness of limb salvage for bone tumours. J Bone Joint Surg Br. 1997 Jul;79(4):558–561. doi: 10.1302/0301-620x.79b4.7687. [DOI] [PubMed] [Google Scholar]
- 10.Robert R.S., Ottaviani G., Huh W.W., Palla S., Jaffe N. Psychosocial and functional outcomes in long-term survivors of osteosarcoma: a comparison of limb-salvage surgery and amputation. Pediatr Blood Cancer. 2010 Jul 1;54(7):990–999. doi: 10.1002/pbc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welker J.A., Henshaw R.M., Jelinek J., Shmookler B.M., Malawer M.M. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000 Dec 15;89(12):2677–2686. doi: 10.1002/1097-0142(20001215)89:12<2677::aid-cncr22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Gherlinzoni F., Picci P., Bacci G. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol. 1992;3(Suppl. 2):S23–S27. doi: 10.1093/annonc/3.suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi N., Matumoto S., Manabe J. New method of evaluating the surgical margin and safety margin for musculoskeletal sarcoma, analysed on the basis of 457 surgical cases. J Cancer Res Clin Oncol. 1995;121(9–10):555–563. doi: 10.1007/BF01197769. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.H., Kang H.G., Kim H.S. MRI-guided navigation surgery with temporary implantable bone markers in limb salvage for sarcoma. Clin Orthop Relat Res. 2010 Aug;468(8):2211–2217. doi: 10.1007/s11999-009-1209-8. Epub 2010 Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cañadell J., Forriol F., Cara J.A. Removal of metaphyseal bone tumours with preservation of the epiphysis. Physeal distraction before excision. J Bone Joint Surg Br. 1994 Jan;76(1):127–132. [PubMed] [Google Scholar]
- 16.Palumbo B.T., Henderson E.R., Groundland J.S. Advances in segmental endoprosthetic reconstruction for extremity tumors: a review of contemporary designs and techniques. Cancer Control. 2011 Jul;18(3):160–170. doi: 10.1177/107327481101800303. [DOI] [PubMed] [Google Scholar]
- 17.Dotan A., Dadia S., Bickels J. Expandable endoprosthesis for limb-sparing surgery in children: long-term results. J Child Orthop. 2010 Oct;4(5):391–400. doi: 10.1007/s11832-010-0270-x. Epub 2010 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscolo D.L., Ayerza M.A., Aponte-Tinao L., Farfalli G. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res. 2008;466:1856–1862. doi: 10.1007/s11999-008-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornicek F.J., Gebhardt M.C., Tomford W.W. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Manfrini M., Tiwari A., Ham J., Colangeli M., Mercuri M. Evolution of surgical treatment for sarcomas of proximal humerus in children: retrospective review at a single institute over 30 years. J Pediatr Orthop. 2011 Jan-Feb;31(1):56–64. doi: 10.1097/BPO.0b013e318202c223. [DOI] [PubMed] [Google Scholar]
- 21.Puri A., Gulia A., Agarwal M., Jambhekar N. Laskar S Extracorporeal irradiated tumor bone: a reconstruction option in diaphyseal Ewing's sarcomas. Indian J Orthop. 2010 Oct;44(4):390–396. doi: 10.4103/0019-5413.69310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya H., Wan S.L., Sakayama K., Yamamoto N., Nishida H., Tomita K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J Bone Joint Surg Br. 2005 Feb;87(2):218–225. doi: 10.1302/0301-620x.87b2.15325. [DOI] [PubMed] [Google Scholar]
- 23.Hariri A., Mascard E., Atlan F. Free vascularised fibular graft for reconstruction of defects of the lower limb after resection of tumour. J Bone Joint Surg Br. 2010 Nov;92(11):1574–1579. doi: 10.1302/0301-620X.92B11.23832. [DOI] [PubMed] [Google Scholar]
- 24.Graci C., Maccauro G., Muratori F., Spinelli M.S., Rosa M.A., Fabbriciani C. Infection following bone tumor resection and reconstruction with tumoral prostheses: a literature review. Int J Immunopathol Pharmacol. 2010 Oct–Dec;23(4):1005–1013. doi: 10.1177/039463201002300405. [DOI] [PubMed] [Google Scholar]
- 25.Jeys L.M., Grimer R.J., Carter S.R., Tillman R.M. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005 Apr;87(4):842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 26.Nathan S.S., Gorlick R., Bukata S. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006 Oct 1;107(7):1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 27.Bacci G., Longhi A., Cesari M., Versari M., Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006 Jun 15;106(12):2701–2706. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 28.Kong C.B., Song W.S., Cho W.H., Oh J.M., Jeon D.G. Local recurrence has only a small effect on survival in high-risk extremity osteosarcoma. Clin Orthop Relat Res. 2012 May;470(5):1482–1490. doi: 10.1007/s11999-011-2137-y. Epub 2011 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehadeh A., Noveau J., Malawer M., Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010 Nov;468(11):2885–2895. doi: 10.1007/s11999-010-1454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai A., Muschler G.F., Lane J.M., Otis J.C., Healey J.H. 1. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998 May;80(5):636–647. doi: 10.2106/00004623-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Henshaw R., Malawer M. Review of endoprosthetic reconstruction in limb-sparing surgery. In: Malawer M.M., Sugarbaker P.H., editors. Musculoskeletal Cancer Surgery Treatment of Sarcomas and Allied Diseases. Kluwer Academic Publishers; Dordrecht: 2001. pp. 381–402. [Google Scholar]
- 32.Rusch V.W. 1. Pulmonary metastasectomy. Current indications. Chest. 1995 Jun;107(Suppl. 6):322S–331S. doi: 10.1378/chest.107.6_supplement.322s. [DOI] [PubMed] [Google Scholar]
- 33.Buddingh E.P., Anninga J.K., Versteegh M.I. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer. 2010 Feb;54(2):216–221. doi: 10.1002/pbc.22293. [DOI] [PubMed] [Google Scholar]


