Abstract
Locally-applied drugs can protect residual hearing following cochlear implantation. The influence of cochlear implantation on drug levels in scala tympani (ST) after round window application was investigated in guinea pigs using the marker trimethylphenlyammonium (TMPA) measured in real-time with TMPA-selective microelectrodes. TMPA concentration in the upper basal turn of ST rapidly increased during implantation and then declined due to cerebrospinal fluid entering ST at the cochlear aqueduct and exiting at the cochleostomy. The TMPA increase was found to be caused by the cochleostomy drilling, if the burr tip partially entered ST. TMPA distribution in the second turn was less affected by implantation procedures. These findings show that basal turn drug levels may be changed during implantation and the changes may need to be considered in the interpretation of therapeutic effects of drugs in conjunction with implantation.
Keywords: Cochleostomy, perforation, cochlear implant, electrode, pharmacokinetics, drug distribution, computational modeling
1. Introduction
Hearing outcomes, especially with respect to music appreciation and speech perception in the presence of background noise, can be improved following cochlear implantation (CI) when residual hearing is protected [von Ilberg et al., 1999; Gantz & Turner, 2003; Gantz et al., 2005; Gstoettner et al., 2008]. Protection strategies are becoming increasingly important as patient selection for CI is widened to include patients with more residual hearing. In addition to soft surgical techniques when performing the cochleostomy [Lehnhardt, 1993; Kiefer et al., 2004; Eshraghi, 2006], and atraumatic electrode design [Gantz & Turner, 2004; Lenarz et al., 2006; Adunka et al., 2004; Baumgartner et al., 2007], residual hearing can be protected with pharmaceuticals such as glucocorticoid steroids following cochlear implant surgery [James et al., 2008; Chang et al., 2009]. Steroids can be administered intratympanically on the round window membrane (RWM) prior to the cochleostomy to inhibit an inflammatory response to the implant, which has resulted in improved cochlear function in humans [Jayawardena et al., 2012] and animals [James et al., 2008; Kiefer et al., 2004; Kiefer et al., 2007; Ye et al., 2007], along with a lowered incidence of dizziness in humans [Enticott et al., 2011]. A study in which function was assessed in non-implanted and implanted groups had previously suggested that inner ear surgery could have a significant effect on intracochlea drug distribution [Eastwood et al., 2010]. In order to understand the best way to apply steroids locally to optimise protection, it is necessary to establish which aspects of the implantation procedure influence drug distribution.
Following intratympanic administration, drug enters the inner ear through both the RWM and in the vicinity of the stapes footplate [King et al., 2011; Salt et al., 2012b]. In the sealed cochlea, substances predominately move in the fluids by passive diffusion driven by concentration gradients. For short-term applications, the influence of longitudinal perilymph flow has been shown to be low [Ohyama et al., 1988; Salt & Ma, 2001]. When a guinea pig cochlea is perforated however, fluid is expelled from the cochleostomy site since cochlear fluidic pressure exceeds atmospheric pressure. This causes an influx of cerebrospinal fluid (CSF) through the cochlea aqueduct (CA), inducing a bulk longitudinal flow between the CA and the cochleostomy. The magnitude of the flow rate is proportionate to the pressure differential across the perforation site. An apical flow of 1.0 μL/min has previously been measured following an apical perforation in a guinea pig [Ohyama et al., 1988]. Thus bulk longitudinal flow plays a more substantial role in drug distribution when the cochlea is perforated.
The traditional method of performing a cochleostomy for CI is to use an otologic drill and diamond burr to perforate the otic capsule anterior/inferior to the RWM to access scala tympani (ST). However studies have shown that direct drilling and suction can cause acoustic trauma resulting in the loss of residual hearing, particularly at low frequencies [Kylén & Arlinger, 1976; Doménech et al., 1989; Pau et al., 2007], and there is some concern this route for CI may potentially cause damage to the spiral ligament and basilar membrane [Lehnhardt, 1993; Briggs et al., 2005]. This has led to the introduction of soft surgical (SS) techniques [Lehnhardt, 1993; Kiefer et al., 2004; Eshraghi, 2006]. A SS technique to open ST is to use a drill to expose the endostium without perforating it, flushing away bone dust with Ringer's solution, and carefully incising the endostium with a micro-lancet knife or needle immediately before implantation, whilst avoiding direct suction in the cochleostomy [Kiefer et al., 2004]. Another SS method gaining clinical interest is to insert the implant directly through the RWM, avoiding drilling of the otic capsule altogether to minimise the potential risk of damage to the delicate intracochlear structures [Skarzynski et al., 2007; Gudis et al., 2012]. Trials are also underway in-vitro to perform the cochleostomy with smart robotic micro-drills [Coulson et al., 2008] or with hand-held CO2 lasers [Fishman et al., 2010; Cipolla et al., 2012].
We have previously shown that cochlear implantation does not significantly influence the distribution of marker Gadolinium within the cochlea at one hour [King et al., 2011], but the affect of the cochleostomy on drug distribution has not been previously quantified. This is an important consideration however in the event that the cochlea has been “preloaded” with drug, as would occur following pre-implantation round window or oval window drug delivery. The method of cochleostomy could potentially alter the intracochlear drug distribution through either fluid leakage or mixing of fluids, the nature of which must be understood if the correct drug dosage is to be delivered to the target structures in order to adequately protect residual hearing following cochlear implantation. Furthermore, we explore the affects of implant insertion in greater detail with direct measures to verify our previous finding that implantation does not substantially redistribute intracochlear drug. This study investigated marker distribution in the scala tympani basal turn (ST1) during these events in real-time using an ion-selective microelectrode sealed into the otic capsule of ST.
2. Materials and Methods
Ion-selective Microelectrode Experiments
2.1. Animal Preparation
The study was conducted in accordance with the policies and recommendations of the United States Department of Agriculture, the National Institute of Health guidelines for the handling and use of laboratory animals, and received approval from the Institutional Animal Care and Use Committee of Washington University under protocols 20070147 and 20100135.
Ten pigmented NIH strain guinea pigs weighing 450–600g, anaesthetized with 100mg/kg sodium thiobutabarbital (Inactin, Sigma, St Louis, MO USA) were used in the study. Anesthesia was maintained with 0.8–1.2% isofluorane in oxygen and the animals were ventilated via a tracheal cannula during surgery with tidal volume set to maintain end-tidal CO2 near 38 mmHg (5%). Heart rate and blood oxygen saturation were monitored continuously by Surgivet pulse-oximeter (Waukesha, WI, USA). Rectal temperature was maintained at 39°C with a thermistor-controlled DC-powered heating blanket. The animals were mounted securely in a head-holder, and the right cochlea exposed by the ventrolateral approach. All experiments were performed as non-recovery procedures.
2.2. Cochleostomy and cochlear implants
The cochleostomy was formed in the basal turn of the guinea pig ST using either SS techniques (n=3) or by using a dental drill fitted with a 0.75mm diameter diamond cutting burr (Sunshine Diamonds, Dr. Hopf GmbH & Co.KG, H001009; n=5) or a 0.52mm diameter carbide burr (Sunshine Carbide, Dr. Hopf GmbH & Co.KG, C1104005; n=2). SS technique entailed thinning the bone initially with a dental burr and using a fine pick to perforate the otic capsule and remove bone fragments. In all cases, the cochleostomy fenestra was made approximately 1.5mm from the lip of the round window membrane.
The cochlear implants used in the study were 100% biocompatible dummy electrodes with three platinum rings (sourced from the Department of Otolaryngology, University of Melbourne). They were cylindrical in shape with 0.4mm diameter and were inserted 2.12 mm (to the edge of the third ring) into the basal turn of scala tympani through the cochleostomy.
2.3. Ion-selective microelectrodes
Ion-selective microelectrodes were sealed in the upper basal turn (n=9) or second turn of ST (n=1) to monitor the perilymph concentration of the marker drug trimethylphenylammonium (TMPA) in real time. The position of the basal turn microelectrode was approximately 3.1 mm from the RWM and the second turn microelectrode was approximately 7.5 mm from the RWM when measured along the scala. The microelectrodes consisted of a double-barrelled glass pipette, one barrel with internal filling fiber and one without. After the glass pipettes were pulled, they were stored in a humidity cabinet at 40°C, 70% humidity overnight. The barrel without fiber was silanized by exposure to dimethyldichlorosilane vapor (Sigma, St. Louis) followed by baking at 140°C for 1 h. The tips were beveled to a diameter of 3–4mm. The silanized, ion barrel was filled with 500 mM KCl and the reference barrel was filled with 500 mM NaCl. TMPA-selective ion exchanger was made from 5% potassium tetrakis(4-chlorophenyl)borate in 2-nitrophenyloctylether (Fluka/ Sigma, NY), that was pre-equilibrated by storage in contact with concentrated TMPA solution. A short column of ion exchanger was drawn into the tip of the ion barrel by suction. The microelectrodes were connected to a high-impedance electrometer through Ag/AgCl wires. Potentials were recorded differentially between the ion and reference barrels of the electrode. Each microelectrode was individually calibrated before use in a series of 5 standards containing 0, 20, 200, 2000 and 20000 mM in a background of artificial perilymph, held at 39° C in a water circulation chamber. TMPA measurements from the animal were collected under computer control, with 10 measurements averaged and stored at 5 s intervals.
2.4. Experimental Procedure
The auditory bulla was accessed using a ventrolateral approach and opened to expose the cochlea. TMPA-selective microelectrodes were sealed into either the upper basal turn (n=9) or second turn (n=1) of ST using methods detailed elsewhere [Ohyama et al., 1988]. Briefly, after the mucosa was removed, the bony wall was thinned with a flap knife. Any fluid present was dried with paper wicks, the bone was allowed to air-dry and then coated with a thin layer of cyanoacrylate glue. A thin layer of two-part Silicone adhesive was applied over the cyanoacrylate glue to make the surface hydrophobic, and a small fenestra (approximately 30–40μm) was made through the adhesives and the bone using a fine pick (0.3mm, 30 degree, Storz N1705 80, Bausch and Lomb Inc). The microelectrode was then positioned in the fenestra and sealed in place with a droplet of cyanoacrylate glue. This method allows the microelectrode to be placed with no subsequent perilymph leakage at the insertion site.
An artificial perilymph solution (127.5mM NaCl, 3.5mM KCl, 25mM NaHCO3, 1.2mM MgCl2, 0.75mM NaH2PO4, 1.3mM CaCl2, 11mM Glucose) containing 20mM TMPA was delivered continuously to the RW niche at a rate of 5μL/min for approximately 30 minutes prior to cochleostomy and implantation. Paper wicks were used to prevent fluid accumulation in the middle ear space. This procedure established a gradient for TMPA along ST with highest levels at the basal entry site near the RWM. The cochleostomy was formed in either the basal turn or second turn of ST using a drill or SS techniques, and ST was implanted with a cochlear implant electrode array.
In some experiments, the timing of events were recorded on a digital audio recording made with Audacity (http://audacity.sourceforge.net/), which was time-synchronized with the automated TMPA measurements.
Computer simulations of the experimental protocol were performed with version 3.081 of the Washington University Cochlear Fluids Simulator, available for download from our website at http://oto.wustl.edu/cochlea/. Parameters for TMPA distribution are incorporated into the program, based on a variety of experimental protocols involving in situ TMPA measurements and measurements of TMPA concentrations in perilymph samples [Salt et al., 2012a].
In-vitro Experiments
In order to visualize the influence of the experimental procedures on fluid movements and substance distribution, we set up a number of in-vitro experiments using solutions marked with fluorescein. In one, a cochlear implant electrode was inserted into a 1.0mm diameter closed-ended, blunt tip glass capillary tube (5 μL, VWR International, Radnor, PA) filled with lactated Ringer's solution. Before the insertion, the fluid region near the tube opening was injected with solution in which 20 mM NaCl was replaced with sodium fluorescein (Sigma-Aldrich, Taufkirchen, Germany). The electrode dummy was inserted into the tube through the Ringers/fluorescein interface using a micromanipulator during which the distribution of fluorescein was recorded with videography.
In some experiments, we used a tube filled with lactated Ringer's which had an opening made in the side wall and fluorescein solution injected at one side of the opening. The tip of the dental burr was inserted into the tube and the change of fluorescein distribution was recorded with videography when the burr was activated. In other experiments, a stream of fluorescein solution was injected from a micropipette in a beaker of lactated Ringer's solution. The influence of dental burrs and implant electrode movements on fluorescein distribution was observed. This allowed us to compare induced changes of distribution under conditions where there were no nearby walls or boundaries.
In all of these experiments the solutions used were simple electrolyte solutions (lactated Ringer's or fluid containing fluorescein) with solution viscosity expected to be close to that of perilymph.
3. Results
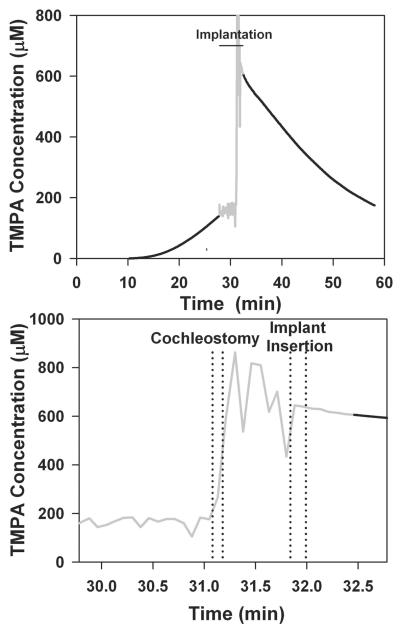
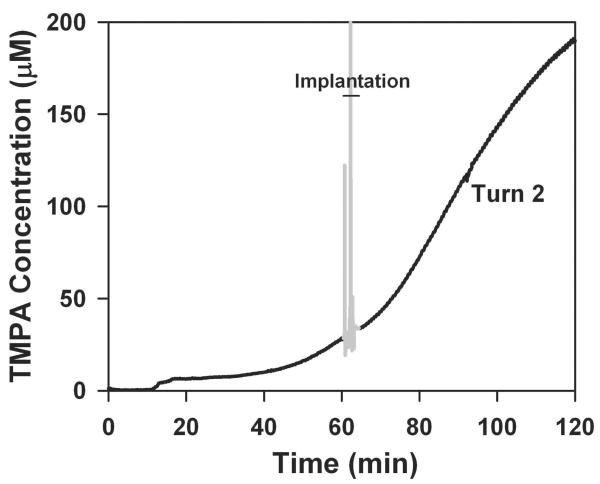
These experiments used TMPA as a marker to represent the distribution of drug applied locally to the RWM approximately 30 minutes before implantation. TMPA concentration can be measured continuously with time throughout a procedure with an ion-selective microelectrode at a fixed location. TMPA time course curves are readily interpreted with computer models to give a realistic representation of how substances are distributed with distance in the ear as a function of time. The experiment in the upper panel of Figure 1 shows the measured time course of TMPA in the upper basal turn of ST before and after cochlear implantation. At zero time, TMPA solution was irrigated across the RWM. After a delay of approximately 10 minutes, during which TMPA diffuses from the RW region to the measurement site, TMPA concentration shows a progressive increase. Throughout the pre-implantation period, there is a longitudinal gradient of TMPA along ST, with the highest concentration near the RWM that progressively spreads further along ST with time. During implantation (shown as gray in Figure 1) the measured concentration becomes noisy and unreliable, due to movements of the experimenter near the high-impedance microelectrode introducing artifactual potentials. Immediately after implantation, when the trace becomes stable again, the measured TMPA level was substantially higher than would be expected by extrapolating the curve prior to implantation. This suggested that the implantation procedure in some manner affected the distribution of TMPA in the cochlea, increasing the TMPA level at the measurement location, which was just apical to the tip of the inserted implant. In order to carefully document the timing of different parts of the implantation procedure, we made digital audio recordings, time-synchronized with the TMPA measurements. The audio recordings were used to distinguish the times when the cochleostomy was drilled and when the cochlear implant electrode was inserted, as shown in the lower panel of Figure 1. Although the measurements are noisy due to the experimental manipulations near the recording microelectrode, it was apparent that the TMPA concentration increased markedly when the cochleostomy was made, rather than at the time of implant insertion. This experiment provided initial evidence against the cochlear implant electrode insertion being the cause of TMPA increase.
Figure 1.
Upper panel: TMPA timecourse recorded from the basal turn of scala tympani following round window irrigation with 20 mM TMPA commencing at zero time, followed by implantation, indicated by the gray, noisy area. The gray, noisy region was when a cochleostomy was drilled and an implant inserted into ST. The noise results from electrical pickup by the high impedance microelectrode due to nearby motion and does not represent actual TMPA concentration change. TMPA irrigation was terminated at the time of implantation, but TMPA solution remained in the RW niche. Following implant insertion the measured TMPA became substantially higher but then declined progressively with time. Lower panel: TMPA concentration during the implantation procedure shown with expanded time scale and with exact event timings derived from audio recordings of the procedure. The lines indicate the start and end of each procedure. Even though the trace is noisy due to movements near the microelectrode, it is apparent that the large increase of concentration is associated with making the cochleostomy, rather than the insertion of the cochlear implant electrode.
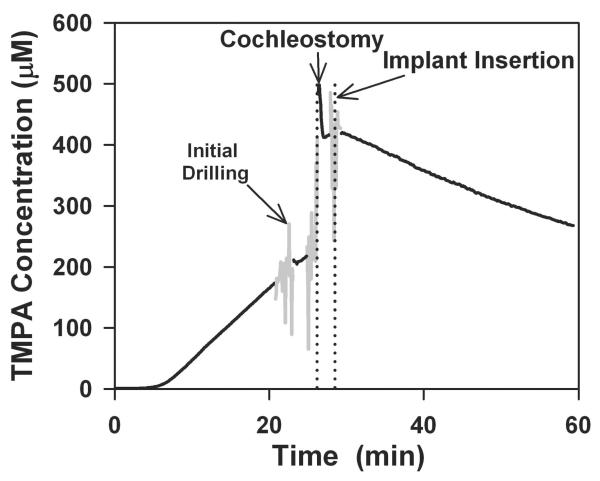
Figure 2 shows an experiment in which the different elements of the implantation procedure were further separated in time to allow undisturbed periods of TMPA recording. Digital audio recordings were again used to keep an accurate record of events with time. In this experiment, the cochleostomy was drilled carefully, pausing the procedure as soon as ST was perforated and fluid appeared. At that time (Figure 2, initial drilling), the progressive TMPA increase was not markedly disturbed. The fenestration was then enlarged sufficiently by drilling to permit implant insertion but pausing the procedure before the implant was inserted. At this time (Figure 2, cochleostomy) the TMPA concentration had increased substantially. Insertion of the implant (Figure 2, Implant insertion) had only a minor influence on the measured TMPA concentration. This experiment clearly demonstrated that it was the act of drilling the cochleostomy, and specifically the tip of the rotating drill entering the fluid of ST, that caused the dramatic increase of concentration at the TMPA measurement site. The percentage increase of TMPA seen as a result of a drilled cochleostomy and implant insertion over 5 individual animals were 193.3%, 156.8%, 612.2%, 406.3% and 191.1% (average 312.0%, SD 174.2%).
Figure 2.
TMPA time course recorded from scala tympani following round window irrigation with 20 mM TMPA, followed by a drilled cochleostomy and implant insertion separated in time to allow periods of undisturbed TMPA recording. The initial (gray) noisy segment coincides with drilling the bone until perilymph was released, but with the perforation too small for the burr or the implant to enter ST. The second gray segment shows the cochleostomy enlarged sufficiently for implant insertion. It was during this procedure that TMPA concentration increased substantially. Implant insertion had negligible immediate influence on TMPA concentration.
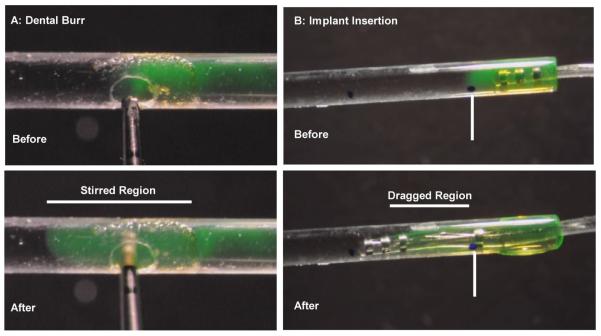
In-vitro studies confirmed that when a dental burr was activated in a narrow tube filled with simple electrolyte solution, it caused a localized intense stirring of the marker (Figure 3A). In contrast, an implant inserted into a closed-ended tube (allowing fluid efflux only at the insertion site) did not appreciably push fluorescent marker in front of it as it was inserted (Figure 3B).
Figure 3.
Influence of procedures demonstrated with fluorescent dye solution in 0.79 mm inner diameter glass capillaries filled with lactated Ringer's (LR) solution and part containing LR with fluorescein. A: Rotating dental burr inserted into the fluid. An entire segment is rapidly stirred and fluorescent dye distribution becomes homogenous within that segment. Regions beyond the stirred segment are unaffected. B: Implant insertion into a closed-end tube in which the only fluid outlet is at the insertion site. Dye solution is not pushed along the tube by the cochlear implant electrode tip but is dragged to some degree by the electrode body.
The entire procedures summarized in Figure 3 are shown in the supplemental video, together with the same procedures shown in open solution (without nearby walls). The dental burr is shown to produce intense stirring and the movement of an implant through the solution is seen to drag nearby solution. In the latter experiment, the apparently viscous nature of the solution is the result of the magnification used and none of the solutions used had viscous properties that differed appreciably from perilymph.
Figure 4 shows a basal turn TMPA recording experiment in which the implantation was performed without the dental burr influencing perilymph. In this experiment, the bone at the cochleostomy site was thinned with a burr but the endostium was opened with a fine pick similar to the approach used in soft surgery. This procedure was more time-consuming but the combined fenestration and implant insertion has almost no influence on the measured TMPA concentration. The slow decline following implantation is again caused by TMPA washout by CSF at the base of ST. The increase of TMPA seen as a result of a cochleostomy made with a pick followed by implant insertion averaged 13.2 % (SD 1.6%, n=2).
Figure 4.
TMPA time course recorded from scala tympani following round window irrigation with 20 mM TMPA, followed by cochleostomy performed with soft surgical techniques and implant insertion.
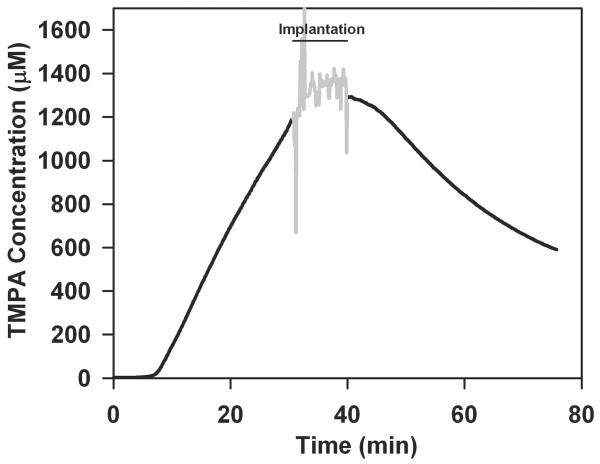
TMPA or drug concentration changes as a result of implantation were most dramatic in the basal turn and were far less when measured at a site further from the implantation site. Figure 5 shows TMPA concentration measured in the second turn of ST (estimated to be 7.5 mm from the base of ST when measured along the scala) during a drilled cochleostomy and cochlear implant electrode insertion; procedures comparable to those in Figures 1 and 2. In this experiment, implantation was performed at 60 mins after TMPA irrigation started to allow a higher concentration to be established at the second turn recording site. Implantation only had a slight influence on the rising TMPA curve, seen as a transient slowing of the rate of increase. The results of this single experiment were consistent with calculated findings (below), and even smaller changes were expected with soft surgical techniques, so the experiment was not repeated. The absence of large changes in the second turn is due to the larger distance and correspondingly prolonged diffusion times for changes in the basal turn to influence the second turn.
Figure 5.
TMPA time course recorded from the second turn of scala tympani following cochleostomy drilling. As it takes longer for TMPA to reach the second turn, the implantation procedure in this experiment was performed 60 minutes after RW irrigation commenced. Cochleostomy drilling and implantation which would induce changes in the basal turn had only minor influence on TMPA concentration measured in the second turn.
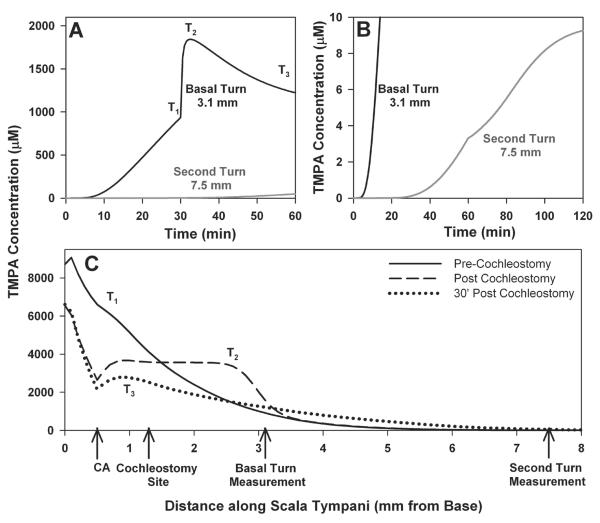
We used our computer model of the inner ear fluid spaces to assess whether the interpretation of TMPA changes during implantation was physically plausible. Figure 6 shows a detailed simulation of the experiments. This includes i) TMPA loading of ST by entry at the round window membrane with a 2 mM round window niche concentration, RW permeability of 5 × 10−7 m/s. ii) diffusion along ST with a diffusion coefficient of 1.01 × 10−9 m2/s combined with an apically-directed volume flow of 8 nl/min in the intact cochlea. iii) Elimination from ST to blood at 35 min half time. iv) Communication with adjacent compartments (spiral ligament, spiral ganglion: 10 min half time). At the time of cochleostomy, the stirring of perilymph by the dental burr was achieved by uniformly distributing the available TMPA over a short segment, as described below, followed by simulation of implant insertion for a 2.25 mm distance over a 20 sec period. This included calculation of the displacement of perilymph from ST at the cochleostomy site with appropriate volume flows and associated TMPA movements along the scala based on the implant and ST dimensions. Since cochlear perforation releases perilymph pressure, a volume flow simulating CSF entry at the cochlear aqueduct and loss at the cochleostomy site at 200 nl/min replaced the apically directed volume flow at the time of cochleostomy. TMPA concentrations are shown as a function of time (Figures 6A, 6B) or with distance along ST (Figure 6C). Figure 6A shows the time course at the basal turn measurement site (3.1 mm from the base) during the initial 30 min TMPA loading period. The TMPA gradient along ST prior to implantation is shown as the curve for time T1 in Figure 6C. Comparison of these curves shows that TMPA concentration at the basal end of ST (near the RW) is predicted to be approximately 8x higher than at the TMPA measurement site. It is this pool of high-TMPA solution that allows the concentration at the measurement site to rise so rapidly during the cochleostomy. The stirring of ST contents during the cochleostomy drilling was implemented by dispersing the available TMPA (taking into account the varying volume with distance along ST) evenly over a distance of 1.6 mm each side of the burr (a distance based on the in vitro experiments). This stirring accounts for the rapid increase (Figure 6A, T2), and is reflected in the more even distribution with distance along ST in Figure 6C, T2. Commencing at the time of cochleostomy, the simulation incorporates CSF entry at the aqueduct with flow towards and leakage at the cochleostomy site. This decreases concentration in the basal region and accounts for the subsequent decline of the time course in Fig 6A. The calculated concentration time course seen in the second turn during these events is also comparable with the measured curve, as shown in Fig 6B for a 60 min pre-implantation loading period. This includes a slight slowing of the rate of increase seen at the time of implantation which may be accounted for by a slow rate of apical volume flow in ST of the intact cochlea that ceases when the scala is perforated at the time of cochleostomy. In the calculations, an apical flow rate of 8 nl/min for the intact cochlea, which stopped at the time of cochleostomy, gives a small inflection of the curve (Figure 6B, second turn), which is more pronounced than that seen in the measured data. This analysis confirms that the measured TMPA time courses could arise from a stirring of the basal region of ST during the cochleostomy procedure.
Figure 6.
TMPA time courses (A and B) and profile with distance along scala tympani (C) calculated with a program that simulates solute movements in the cochlear fluids. T1, T2 and T3 show different time points in panel A for which the concentration profile with distance is shown in panel C. Prior to cochleostomy a substantial gradient for TMPA exists along ST (Trace T1 in panel C). To simulate the fluids stirring resulting from a drilled cochleostomy, solute over the region up to 1.6 mm each side of the cochleostomy location was summed and distributed equally, equivalent to fully mixing the solute in the basal 2.9 mm of ST. As the solution at the measurement site (at 3.1 mm from the base) becomes mixed with higher concentration, more basal solution, concentration measured at that site rapidly rises (Panel A). Panel B shows calculated time courses scaled to see the second turn measurement site and for a simulated cochleostomy occurring at 60 mins after TMPA application commences (comparable to the protocol in Figure 5). CA indicates the location in the model where the cochlear aqueduct enters ST.
4. Discussion
This is the first study in which the alteration of perilymph marker distribution during cochleostomy and cochlear implantation has been assessed by direct, real-time measurements of concentration. Our initial results showed a substantial rise in concentration at the measurement site in the upper basal turn of ST at the time of implantation. This was first thought to be due to the cochlear implant electrode pushing higher concentration solution from the region near the cochleostomy site apicalwards to the recording site during insertion. More detailed experiments, however, revealed that this was not the case. The increase was found to be caused by the act of drilling the cochleostomy, with the perilymph near the cochleostomy site being stirred when a portion of the rotating burr entered the scala. The concentration rise caused by the cochleostomy procedure was immediately followed by a progressive decline in concentration. This was driven by the washout of TMPA-containing perilymph from the ST basal turn by CSF flow from the cochlear aqueduct (CA) to the cochleostomy site, and due to TMPA diffusing to regions of lower concentration. Conversely, the rapid concentration increase did not occur in the second turn of ST when the cochleostomy was drilled, which is over 6 mm from the cochleostomy site, or in the basal turn when soft surgical techniques were used to make the cochleostomy. In-vitro experiments confirmed the marker was intensely stirred by a dental burr, creating complex flow and vortices, rapidly re-distributing the marker a short distance in both directions along the glass tube when the rotating dental burr came into contact with the fluid (Figure 3A). These data therefore show that implantation procedures can have a substantial and immediate influence on drug levels in the basal turn, which could in turn influence functional measurements if drug level plays a critical role in determining functional state. Functional measurements can also be influenced by other factors such as hearing loss associated with the surgical procedures.
Even though performing the cochleostomy by drilling resulted in a wider drug distribution along ST than using SS techniques, which may be advantageous for expediting the delivery of protective drugs along the cochlea, drilling has several known disadvantages. These include the increased risk of acoustic trauma and the high likelihood of bone dust entering scala tympani, which may be a nidus for more extensive fibrosis and undesired osteoneogenesis. To avoid this, the “ideal” cochleostomy is performed by “blue-lining” the endostium that entails thinning the bone with the burr and then opening the endostium with a pick, as was performed in some of these experiments. In practice, the endostium is often breached and the waist of the drill can enter the scala as we have modeled here. So we conclude that drilling the cochleostomy is not advisable, however inadvertent scalar entry with the drill may increase the reach of protective drugs if they are already present within the inner ear, which could be advantageous if this surgical event occurs.
Based on our observations from this study, we speculate that surgical drilling could potentially be used to widen the distribution of drug in other otologic surgeries and in particular stapedotomy. Fenestration of the stapes footplate with a micro-drill is a popular technique in stapedotomy, where the technique is analogous to that emulated here; many surgeons will introduce the burr into the stapedotomy to its waist in order to ensure consistency of the size of the fenestra. We have recently demonstrated the efficacy of stapedial drug delivery (King et al., 2011; Salt et al., 2012), so if in the future this route were used to pre-load the vestibule prior to stapes surgery, drill-assisted stapedotomy would be expected to broaden the distribution of drug already present within the vestibular perilymph.
The act of inserting a cochlear implant electrode into the cochlea did not cause a rapid re-distribution of intracochlear drug in ST. The volume of perilymph displaced during electrode insertion into the basal turn of ST is equivalent to the volume of the electrode. This causes fluid to be expelled from the cochleostomy and possibly through the CA, carrying drug-laden perilymph basalward since there is not an outlet at the apex. It was evident in the in-vitro experiments that fluorescein was expelled from the open end of the tube during electrode insertion (in the opposite direction to the movement of the electrode), and the electrode tip passed through the fluorescein/Ringers interface without pushing a bolus of fluorescein in front of it (Figure 3B). Conversely, when the closed end of the tube was perforated and the experiment repeated, fluorescein was pushed in front of the electrode tip during insertion due to the fluorescein being carried with the body of fluid leaking from the distal perforation (data not shown). These experiments demonstrate that bulk longitudinal flow is established toward the perforation site during electrode insertion, and the electrode tip does not push a bolus of drug in front of it when there is no distal perforation present.
Even though the bulk of the fluorescein was expelled from the open end of the tube during electrode insertion, we observed that a small amount was also being dragged along with the electrode array around its circumference (Figure 3B). This is presumably due to fluid viscosity and surface tension of fluids in small spaces. Other experiments (shown in supplemental video) confirmed the electrode drags fluorescein when the electrode was advanced back and forth through a stream of it. This may become an important consideration if the electrode is inserted, removed, and re-inserted during cochlear implantation, since presumably this would drag drug with it, altering intracochlear drug distribution.
The ramification of intracochlear drug being expelled with perilymph from the cochleostomy is that there may be lower levels of drug present in the basal turn than anticipated. However it is expected the local drug losses in scala tympani will be partially replenished by radial transfer from scala vestibuli, due to the concentration gradient, when the vestibule is loaded with drug. Our previous studies (King et al., 2011; Salt et al., 2012b) show that in the guinea pig, drug directly enters the oval window, likely through the annular ligament of the stapediovestibular joint, following intratympanic application. This can heavily load the vestibule with drug, making it act like a drug repository. This has also been shown in humans and rats (Zou et al., 2005).
Both the modelling and measurement in the second turn show that drug levels following local application to the round window niche are substantially lower in the second turn than in the basal turn. This is consistent with prior studies that have shown substantial longitudinal gradients of drug following local applications (Mynatt et al., 2006; Plontke et al, 2007; Plontke et al., 2008). It is also consistent with Chang et al., (2009) who reported that longer waiting times between drug application and implantation achieved the best protection of function (optimal at 2 hours), assessed by ABR in guinea pigs. So even with the presence of drug gradients, therapeutic levels can be achieved in the second turn. There is greater concern if a primary goal of the drug therapy is to help preserve low frequency (apical) sensitivity of the ear following implantation. As drug distribution is dominated by diffusion it may not be possible to achieve therapeutic drug levels in apical regions after short application times. In the human, drug levels reaching apical regions are likely to be even lower than the guinea pig as ST is almost twice the length in the human. It is presently unknown whether drugs must reach the apex to protect low frequency hearing or whether basal applications would be effective. If therapeutic drug levels at the apex are necessary, alternative strategies, such as delivering drugs from the implant itself, may be required.
The experimental data showed considerable variability in the absolute levels of TMPA found in perilymph following local application to the round window niche. This is due to the high variability of RW membrane permeability which has been reported in prior studies (Salt and Ma, 2001; Mynatt et al., 2006). Intracochlear drug applications would overcome this source of variability.
This paper demonstrates that the method of performing the cochleostomy influences the distribution of drug present in ST1 more than the insertion of the electrode array. We found that the distribution of drug in ST1 can be expanded by the use of a surgical drill entering the scala, however this is unlikely to assist with the preservation of residual hearing when steroids are used since the disturbance was not observed in the upper cochlear turns where the hair cells used for speech recognition reside. The stirring of perilymph could potentially be used to advantage in other clinical contexts, such as stapedectomy where a protective drug delivered to the oval window could be more widely distributed throughout the vestibule after stapedotomy.
Supplementary Material
Acknowledgements
The authors would like to thank Ruth Gill for preparing the figures, Dr Tim Hullar for his suggested experiments. This study was supported by NIH/NIDCD research grant DC 01368 and the National Health and Medical Research Council of Australia Project grant 1007948.
References
- Adunka O, Kiefer J, Unkelbach MH, Lehnert T, Gstoettner W. Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope. 2004;114(7):1237–1241. doi: 10.1097/00005537-200407000-00018. [DOI] [PubMed] [Google Scholar]
- Baumgartner WD, Jappel A, Morera C, Gstöttner W, Müller J, Kiefer J, Van De Heyning P, Anderson I, Nielsen SB. Outcomes in adults implanted with the FLEXsoft electrode. Acta Otolaryngol. 2007;127(6):579–586. doi: 10.1080/00016480600987784. [DOI] [PubMed] [Google Scholar]
- Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125(8):870–876. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- Chang A, Eastwood H, Sly D, James D, Richardson R, O'Leary S. Factors influencing the efficacy of round window dexamethasone protection of residual hearing post-cochlear implant surgery. Hear Res. 2009;255(1–2):67–72. doi: 10.1016/j.heares.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Iyer P, Dome C, Welling DB, Bush ML. Modification and comparison of minimally invasive cochleostomy techniques: A pilot study. Laryngoscope. 2012;122(5):1142–7. doi: 10.1002/lary.23231. [DOI] [PubMed] [Google Scholar]
- Coulson CJ, Taylor RP, Reid AP, Griffiths MV, Proops DW, Brett PN. An autonomous surgical robot for drilling a cochleostomy: preliminary porcine trial. Clin Otolaryngol. 2008;33(4):343–347. doi: 10.1111/j.1749-4486.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- Doménech J, Carulla M, Traserra J. Sensorineural high-frequency hearing loss after drill-generated acoustic trauma in tympanoplasty. Arch Otorhinolaryngol. 1989;246:280–282. doi: 10.1007/BF00463575. [DOI] [PubMed] [Google Scholar]
- Eastwood H, Pinder D, James D, Chang A, Galloway S, Richardson R, O'Leary S. Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation. Hear Res. 2010;259:24–30. doi: 10.1016/j.heares.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Enticott JC, Eastwood HT, Briggs RJ, Dowell RC, O'Leary SJ. Methylprednisolone Applied Directly to the Round Window Reduces Dizziness after Cochlear Implantation: A Randomized Clinical Trial. Audiol Neurotol. 2011;16:289–303. doi: 10.1159/000322137. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA. Prevention of cochlear implant electrode damage. Current Opinion Otolaryngol Head Neck Surg. 2006;14:323–328. doi: 10.1097/01.moo.0000244189.74431.df. [DOI] [PubMed] [Google Scholar]
- Fishman AJ, Moreno LE, Rivera A, Richter CP. CO(2) laser fiber soft cochleostomy: development of a technique using human temporal bones and a guinea pig model. Lasers Surg Med. 2010;42(3):245–256. doi: 10.1002/lsm.20902. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113(10):1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124(4):344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115(5):796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, van de Heyning P, O'Connor AF, Morera C, Sainz M, Vermeire K, Mcdonald S, Cavallé L, Helbig S, Valdecasas JG, Anderson I, Adunka OF. Electric acoustic stimulation of the auditory system: results of a multi-centre investigation. Acta Otolaryngol. 2008;128(9):968–975. doi: 10.1080/00016480701805471. [DOI] [PubMed] [Google Scholar]
- Gudis DA, Montes M, Bigelow DC, Ruckenstein MJ. The Round Window: Is it the “Cochleostomy” of Choice? Experience in 130 Consecutive Cochlear Implants. Otol Neurotol. 2012;33(9):1497–501. doi: 10.1097/MAO.0b013e31826a52c7. [DOI] [PubMed] [Google Scholar]
- James DP, Eastwood H, Richardson RT, O'Leary SJ. Effects of round window dexamethasone on residual hearing in a Guinea pig model of cochlear implantation. Audiol Neurootol. 2008;13(2):86–96. doi: 10.1159/000111780. [DOI] [PubMed] [Google Scholar]
- Jayawardena J, Kuthubutheen J, Rajan G. Hearing preservation and hearing improvement after reimplantation of pediatric and adult patients with partial deafness: a retrospective case series review. Otol Neurotol. 2012 Jul;33(5):740–744. doi: 10.1097/MAO.0b013e318255dd91. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, Baumgartner W, Pok SM, Tillein J, Ye Q, von Ilberg C. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124(3):272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Ye Q, Tillein J, Adunka O, Arnold W, Gstöttner W. Protecting the cochlea during stapes surgery: is there a role for corticosteroids? Adv Otorhinolaryngol. 2007;65:300–307. doi: 10.1159/000098849. [DOI] [PubMed] [Google Scholar]
- King EB, Salt AN, Eastwood HT, O'Leary SJ. Direct Entry of Gadolinium into the Vestibule Following Intratympanic Applications in Guinea Pigs and the Influence of Cochlear Implantation. JARO. 2011;12(6):741–751. doi: 10.1007/s10162-011-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylén P, Arlinger S. Drill-generated noise levels in ear surgery. Acta Otolaryngol (Stockh) 1976;82:402–409. doi: 10.3109/00016487609120925. [DOI] [PubMed] [Google Scholar]
- Lenarz T, Stover T, Buechner A, Paasche G, Briggs R, Risi F, Pesch J, Battmer RD. Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurootol. 2006;11(suppl 1):34–41. doi: 10.1159/000095612. [DOI] [PubMed] [Google Scholar]
- Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;41(7):356–359. [PubMed] [Google Scholar]
- Mynatt R, Hale SA, Gill RM, Plontke SKR, Salt AN. Demonstration of a longitudinal concentration gradient along scala tympani by sequential sampling of perilymph from the cochlear apex. J. Assoc. Res. Otolaryngol. 2006;7:182–193. doi: 10.1007/s10162-006-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea-pig cochlea. Hear Res. 1988;35(2–3):119–129. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Pau HW, Just T, Bornitz M, Lasurashvilli N, Zahnert T. Noise exposure of the inner ear during drilling a cochleostomy for cochlear implantation. Laryngoscope. 2007;117(3):535–540. doi: 10.1097/MLG.0b013e31802f4169. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Mynatt R, Gill RM, Salt AN. Concentration gradient along scala tympani following the local application of gentamicin to the round window membrane. Laryngoscope. 2007;117:1191–1198. doi: 10.1097/MLG.0b013e318058a06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SK, Biegner T, Kammerer B, Delabar U, Salt AN. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear.Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- Salt AN, Hartsock JJ, Gill RM, Piu F, Plontke SK. Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol. 2012a;13(6):771–783. doi: 10.1007/s10162-012-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN, King EB, Hartsock JJ, Gill RM, O'Leary SJ. Marker Entry into Vestibular Perilymph via the Stapes Following Applications to the Round Window Niche of Guinea Pigs. Hear Res. 2012b;283(1–2):14–23. doi: 10.1016/j.heares.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, Piotrowska A, Anderson I. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol. 2007;127(1):41–48. doi: 10.1080/00016480500488917. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Stürzebecher E, Klinke R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- Ye Q, Tillein J, Hartmann R, Gstoettner W, Kiefer J. Application of a Corticosteroid (Triamcinolon) Protects Inner Ear Function after Surgical Intervention. Ear and Hearing. 2007;28(3):361–369. doi: 10.1097/01.aud.0000261655.30652.62. [DOI] [PubMed] [Google Scholar]
- Zou J, Pyykkö I, Bjelke B, Dastidar P, Toppila E. Communication between the Perilymphatic Scalae and Spiral Ligament Visualized by in vivo MRI. Audiol Neurotol. 2005a;10:145–152. doi: 10.1159/000084024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.