Abstract
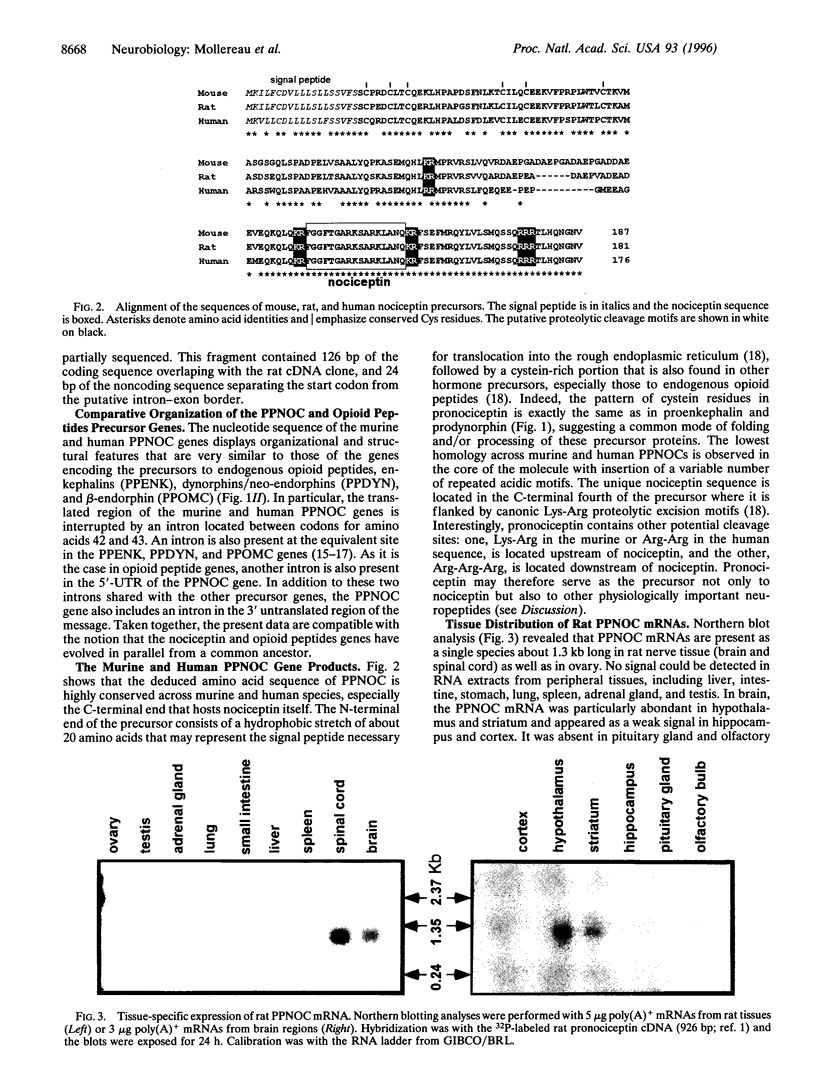
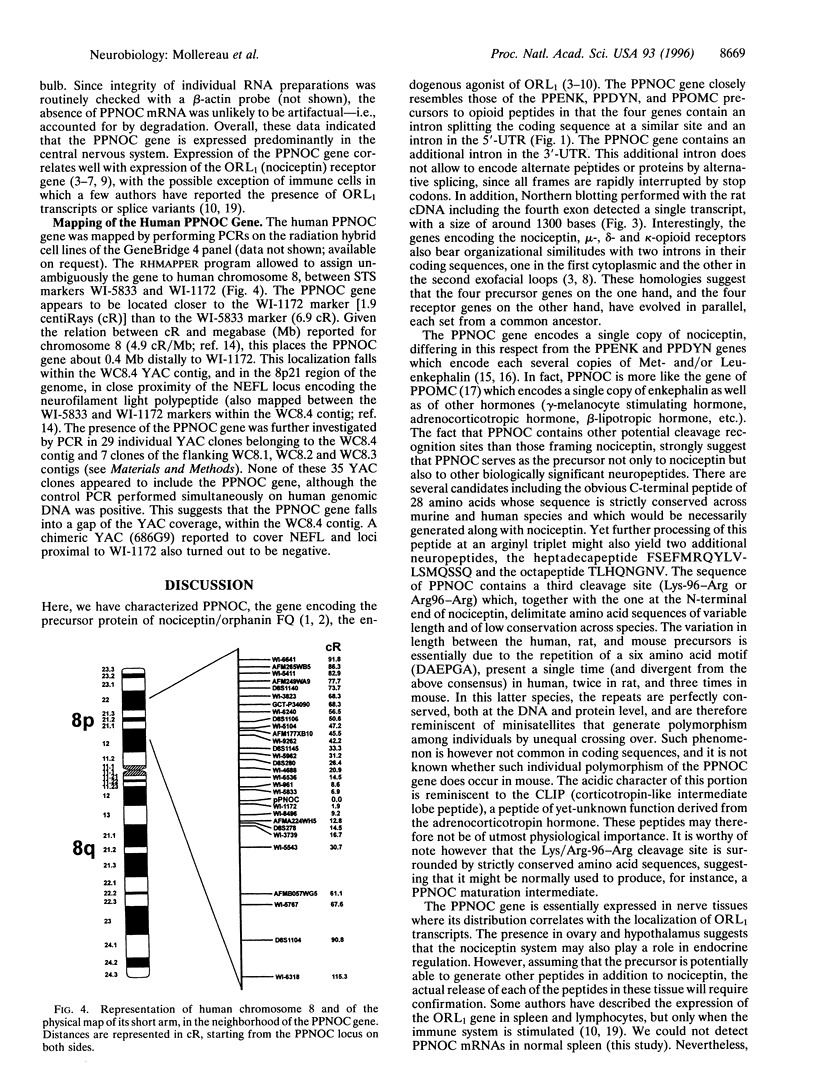
Nociceptin (orphanin FQ), the newly discovered natural agonist of opioid receptor-like (ORL1) receptor, is a neuropeptide that is endowed with pronociceptive activity in vivo. Nociceptin is derived from a larger precursor, prepronociceptin (PPNOC), whose human, mouse, and rat genes we have now isolated. The PPNOC gene is highly conserved in the three species and displays organizational features that are strikingly similar to those of the genes of preproenkephalin, preprodynorphin, and preproopiomelanocortin, the precursors to endogenous opioid peptides, suggesting the four genes belong to the same family-i.e., have a common evolutionary origin. The PPNOC gene encodes a single copy of nociceptin as well as of other peptides whose sequence is strictly conserved across murine and human species; hence it is likely to be neurophysiologically significant. Northern blot analysis shows that the PPNOC gene is predominantly transcribed in the central nervous system (brain and spinal cord) and, albeit weakly, in the ovary, the sole peripheral organ expressing the gene. By using a radiation hybrid cell line panel, the PPNOC gene was mapped to the short arm of human chromosome 8 (8p21), between sequence-tagged site markers WI-5833 and WI-1172, in close proximity of the locus encoding the neurofilament light chain NEFL. Analysis of yeast artificial chromosome clones belonging to the WC8.4 contig covering the 8p21 region did not allow to detect the presence of the gene on these yeast artificial chromosomes, suggesting a gap in the coverage within this contig.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunzow J. R., Saez C., Mortrud M., Bouvier C., Williams J. T., Low M., Grandy D. K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994 Jun 27;347(2-3):284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Chen Y., Fan Y., Liu J., Mestek A., Tian M., Kozak C. A., Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994 Jun 27;347(2-3):279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kato S., Mori K., Nishi M., Takeshima H., Iwabe N., Miyata T., Houtani T., Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994 Apr 18;343(1):42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Halford W. P., Gebhardt B. M., Carr D. J. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 1995 Jun;59(1-2):91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- Horikawa S., Takai T., Toyosato M., Takahashi H., Noda M., Kakidani H., Kubo T., Hirose T., Inayama S., Hayashida H. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983 Dec 8;306(5943):611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Hudson T. J., Stein L. D., Gerety S. S., Ma J., Castle A. B., Silva J., Slonim D. K., Baptista R., Kruglyak L., Xu S. H. An STS-based map of the human genome. Science. 1995 Dec 22;270(5244):1945–1954. doi: 10.1126/science.270.5244.1945. [DOI] [PubMed] [Google Scholar]
- Lachowicz J. E., Shen Y., Monsma F. J., Jr, Sibley D. R. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem. 1995 Jan;64(1):34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P., Butour J. L., Guillemot J. C., Ferrara P., Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995 Oct 12;377(6549):532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mollereau C., Parmentier M., Mailleux P., Butour J. L., Moisand C., Chalon P., Caput D., Vassart G., Meunier J. C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994 Mar 14;341(1):33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Nishi M., Takeshima H., Mori M., Nakagawara K., Takeuchi T. Structure and chromosomal mapping of genes for the mouse kappa-opioid receptor and an opioid receptor homologue (MOR-C). Biochem Biophys Res Commun. 1994 Dec 15;205(2):1353–1357. doi: 10.1006/bbrc.1994.2814. [DOI] [PubMed] [Google Scholar]
- Noda M., Teranishi Y., Takahashi H., Toyosato M., Notake M., Nakanishi S., Numa S. Isolation and structural organization of the human preproenkephalin gene. Nature. 1982 Jun 3;297(5865):431–434. doi: 10.1038/297431a0. [DOI] [PubMed] [Google Scholar]
- Reinscheid R. K., Nothacker H. P., Bourson A., Ardati A., Henningsen R. A., Bunzow J. R., Grandy D. K., Langen H., Monsma F. J., Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995 Nov 3;270(5237):792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Saito Y., Maruyama K., Saido T. C., Kawashima S. N23K, a gene transiently up-regulated during neural differentiation, encodes a precursor protein for a newly identified neuropeptide nociceptin. Biochem Biophys Res Commun. 1995 Dec 14;217(2):539–545. doi: 10.1006/bbrc.1995.2809. [DOI] [PubMed] [Google Scholar]
- Sossin W. S., Fisher J. M., Scheller R. H. Cellular and molecular biology of neuropeptide processing and packaging. Neuron. 1989 May;2(5):1407–1417. doi: 10.1016/0896-6273(89)90186-4. [DOI] [PubMed] [Google Scholar]
- Walter M. A., Spillett D. J., Thomas P., Weissenbach J., Goodfellow P. N. A method for constructing radiation hybrid maps of whole genomes. Nat Genet. 1994 May;7(1):22–28. doi: 10.1038/ng0594-22. [DOI] [PubMed] [Google Scholar]
- Wang J. B., Johnson P. S., Imai Y., Persico A. M., Ozenberger B. A., Eppler C. M., Uhl G. R. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994 Jul 4;348(1):75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Minnerath S. R., Roy S., Ramakrishnan S., Loh H. H. Expression of alternate forms of brain opioid 'orphan' receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Brain Res Mol Brain Res. 1995 Sep;32(2):342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]



