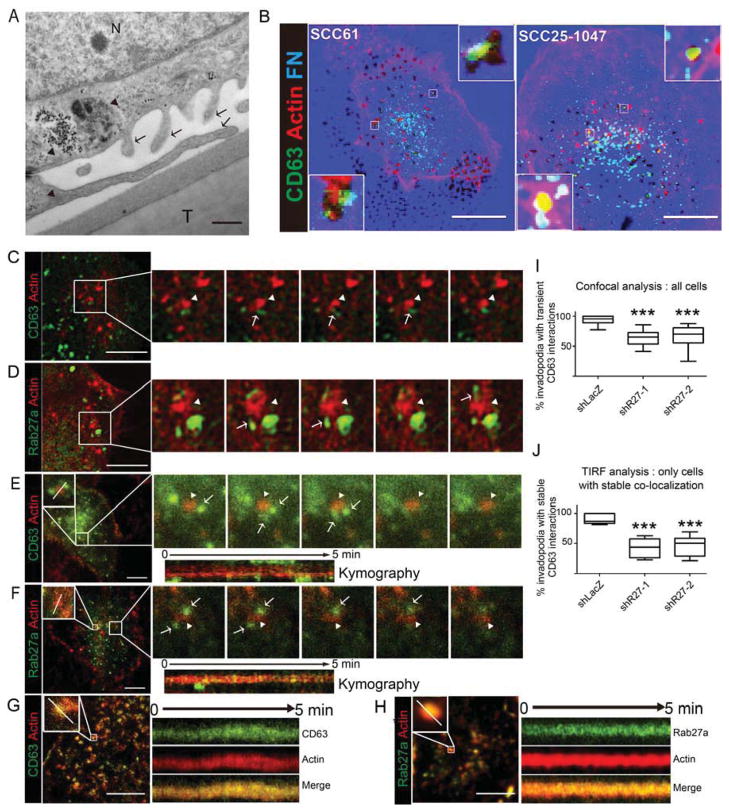
Figure 1. MVE are recruited to invadopodia sites.
(A) SCC61 cells cultured on crosslinked gelatin-coated Transwell filters. Arrows point to invadopodia. Arrowheads point to MVE and MVE-containing autophagolysosomes docked near invadopodia. N = nucleus. T = Transwell filter. (B) Confocal images of cells expressing GFP-CD63 (green) cultured on Alexa-633-fibronectin (FN)-coated gelatin (blue) and stained with rhodamine phalloidin (red) to detect actin filaments. Dark spots in the FN images indicate degradation. Scale bars=10 μm. (C–F) SCC25-H1047R cells stably expressing F-tractin (red) were transfected with GFP-CD63 (C,E) or GFP-Rab27a (D,F) (green) and cultured for 24 h on FN-coated gelatin plates for live confocal microscopy (C,D) or on FN-coated plates for live TIRF microscopy (E–H). Frame rates are 1 per 0.97 sec (confocal) or 1 per 2.8 sec (TIRF). Sequential frames show transient and tubular interactions of green GFP-CD63- or GFP-Rab27a-positive vesicular structures (arrows) with invadopodia (arrowheads). Scale bars=20 μm (C,D) or 10 μm (E,F,G,H). (G,H): TIRF movies showing stable colocalization of GFP-CD63 and GFP-Rab27a with invadopodia. Kymographs show examples of transient (E,F) and stable (G,H) interactions between exosome markers and invadopodia. (I,J) Percent invadopodia per cell with transient (I) or stable (J) interactions with GFP-CD63-positive endosomes in control (shLacZ) and Rab27a-KD (shR27) cells. Data plotted as box-and-whiskers plots where the median is represented with a line, the box represents the 25–75 percentile, and error bars show the 5–95 percentile. ***p<0.001. N≥ 10 cells from 10 movies from ≥3 independent experiments.