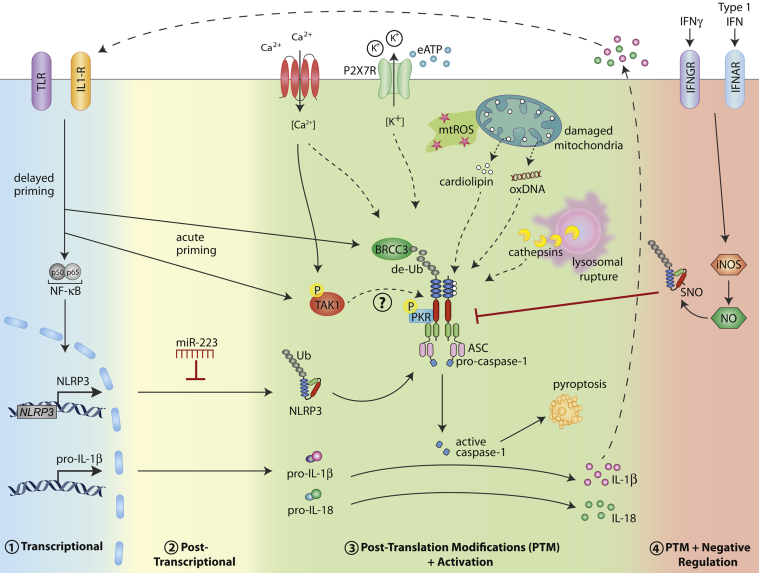
Figure 1.
Multiple levels of NLRP3 inflammasome regulation. Various types of cellular stress, including intracellular ROS production, lysosomal leakage, and ion fluxes (K+ efflux and Ca2+ influx), can trigger activation of the NLRP3 inflammasome. The events leading to NLRP3 activation appear to involve pathways mediating mitochondrial damage and the release of mitochondrial content into the cytosol [eg, oxidized DNA (oxDNA) and cardiolipin]. In addition, high levels of eATP activate NLRP3 after eATP binding the P2X7 receptor. Activation of NLRP3 leads to maturation and release of IL-1β and IL-18 cytokines after caspase-1–dependent proteolysis. In addition, capase-1 activation results in cell death via pyroptosis. The activation status of NLRP3 is modulated on multiple levels, to avoid aberrant activation. 1) In macrophages, NLRP3 (and pro-IL-1β) protein levels are controlled by a delayed transcriptional priming step mediated via activation of PRRs and cytokine receptors upstream of the transcription factor NFκB. 2) In resting myeloid cells, NLRP3 is negatively regulated via miR-223 at the post-transcriptional level. 3) Acutely, NLRP3 can be activated by BRCC3-dependent deubiquitination (de-Ub). The kinase activity of Syk, PKR, and TAK1 all play a role in NLRP3 activation, suggesting phosphorylation of NLRP3 may also be an activation requirement. 4) Type I IFN or IFNGR signaling leads to production of NO via activation of inducible nitric oxide synthase (iNOS). NO can inhibit NLRP3 inflammasome formation via SNO modification.