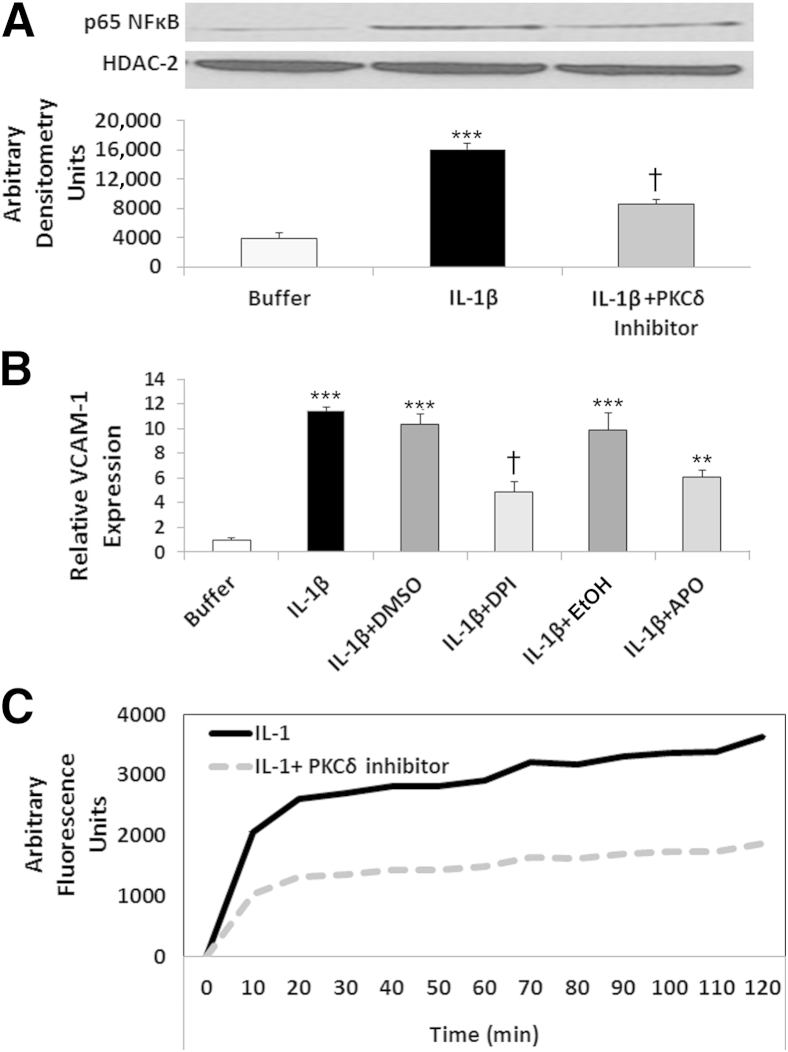
Figure 8.
Role for PKCδ in IL-1β–mediated ROS production and activation of the redox-sensitive transcription factor NF-κB. A: IL-1β–mediated p65 NF-κB translocation is PKCδ dependent. PMVECs were treated with buffer or 10 U/mL IL-1β ± PKCδ inhibitor. Nuclear extracts, prepared after 15 minutes of incubation, were probed for the presence of p65 NF-κB by Western blotting. Representative blots are shown, with quantitation. HDAC-2 is a marker for nuclear fractions. B: Regulatory role for ROS in IL-1β–mediated VCAM-1 expression in PMVECs. VCAM-1 expression was measured by cell-surface ELISA. Constitutive expression of VCAM-1 (buffer) was normalized to 1, and expression in response to IL-1β treatment ± the ROS inhibitors DPI (10 μmol/L) or apocynin (APO; 500 μmol/L) or appropriate vehicle controls (DMSO and EtOH, respectively) was compared. C: PKCδ inhibition attenuates ROS production in IL-1β–treated PMVECs. CM-H2DCFDA–loaded PMVEC monolayers were treated with 10 U/mL IL-1β ± 2 μmol/L PKCδ inhibitor. Controls (Dulbecco’s modified Eagle’s medium) exhibited no change in oxidation of the probe, and H2O2 confirmed presence of the probe (data not shown). Graph in C is representative of four separate experiments run in triplicate (P < 0.03 IL-1β versus IL-1β+PKCδ inhibitor). Data are expressed as means ± SEM (A and B). n = 5 (A); n = 4 (B). ∗∗∗P < 0.001 IL-1β–treated PMVECs versus buffer-treated PMVECs; †P < 0.001 IL-1β–treated PMVECs versus IL-1β+PKCδ inhibitor–treated PMVECs (A). ∗∗∗P < 0.001 buffer versus IL-1β, IL-1β+DMSO, and IL-1β+EtOH; †P < 0.001 IL-1β+DPI versus IL-1β and IL-1β+DMSO; and ∗∗P < 0.001 IL-1β+APO versus IL-1β and IL-1β+EtOH (B).