Figure 1.
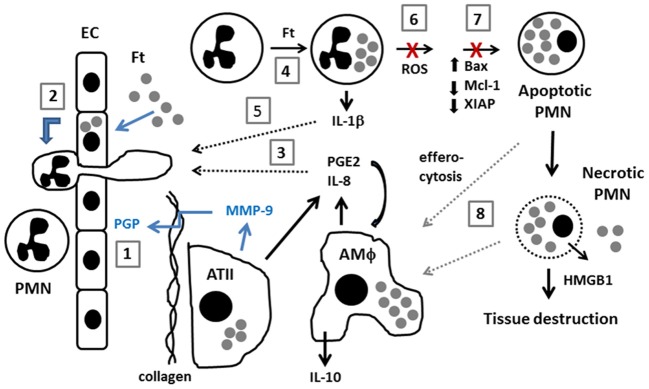
Model of neutrophil dynamics in the F. tularensis-infected lung. Inhaled F. tularensis rapidly infects alveolar type II (ATII) cells and macrophages (AMΦ) (Gentry et al., 2007). (1) MMP-9, likely secreted by ATII cells, cleaves collagen, generating PGP which directly stimulates PMN recruitment from the bloodstream (Malik et al., 2007). (2) PMN migration is also stimulated by direct infection of pulmonary endothelial cells (EC) by an IL-8 and MCP-1-independent mechanism (Moreland et al., 2009). (3) F. tularensis stimulates release of PGE2 and IL-8 from AMΦ and ATII cells (Gentry et al., 2007; Woolard et al., 2007). PGE2 stimulates macrophage production of IL-10 (Hunt et al., 2012). (4,5) F. tularensis infects PMNs and upregulates IL-1β (Schwartz et al., 2013). The extent to which PGE2, IL-8 and IL-1β contribute to PMN chemotaxis and phenotypic modulation during tularemia remains to be determined (dotted black arrows). (6,7) F. tularensis inhibits PMN NADPH oxidase activity and prevents changes in gene expression that are critical for constitutive and phagocytosis-induced apoptosis (McCaffrey and Allen, 2006; McCaffrey et al., 2010; Schwartz et al., 2012a, 2013). (8) Efferocytosis of apoptotic PMNs is critical for control of infection and resolution of inflammation. Defects in apoptosis favor PMN necrosis, and subsequent release of cytotoxic cell components, and danger molecules such as HMGB1 exacerbate tissue destruction. Recent data suggest that efferocytosis and/or clearance of necrotic cell debris may be impaired (Mares et al., 2011) (dotted gray arrows).
