FIGURE 10.
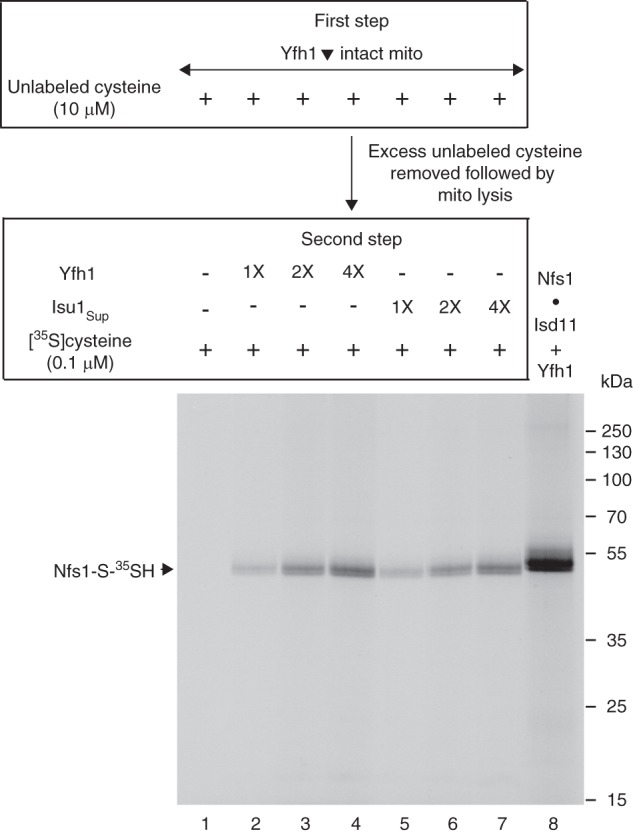
Addition of purified Yfh1 or Isu1Sup exposes the substrate-binding sites of Nfs1 for effective interaction with cysteine in mitochondrial lysate. Mitochondria were isolated from the Gal-Yfh1 strain after an 18 h depletion of Yfh1 in galactose-free medium. Intact Yfh1-depleted mitochondria (Yfh1▾; 200 μg of proteins) in HS buffer were incubated at 30 °C for 10 min in the presence of unlabeled cysteine (10 μm) (first step). After dilution with HS buffer, mitochondria were recovered by centrifugation, removing excess and free cysteine. The mitochondrial pellet was resuspended in 20 mm Hepes/KOH, pH 7.5, and membranes were ruptured. The lysate thus obtained was supplemented with purified Yfh1 or Isu1Sup (1× = 50 ng) as indicated. The reaction mixtures were adjusted to 100 μl with final concentrations of 20 mm Hepes/KOH, pH 7.5, 0.1 μm [35S]cysteine (10 μCi), 0.15 m NaCl, 40 mm KOAc, and 10 mm Mg(OAc)2, and samples were incubated at 30 °C for 15 min (second step) (lanes 1–7). As control (lane 8), a mixture of purified Nfs1·Isd11 complex (100 ng) and Yfh1 (100 ng) was incubated with 0.1 μm [35S]cysteine (5 μCi) and 0.15 mm PLP in a final volume of 50 μl. Proteins were precipitated with 10% TCA and analyzed by nonreducing SDS-PAGE followed by autoradiography.
