FIGURE 3.
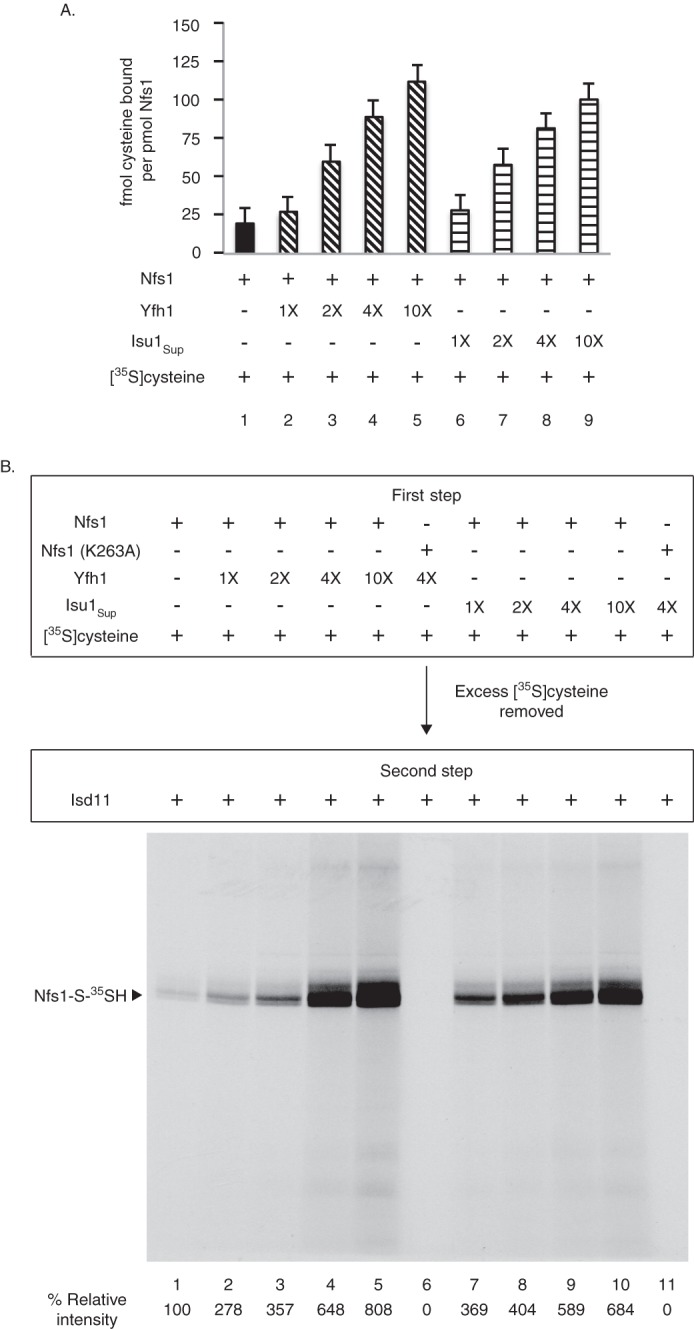
Yfh1 or Isu1Sup stimulates cysteine binding to purified Nfs1. A, as indicated, Nfs1 (100 ng) was mixed with Yfh1 or Isu1Sup (1× = 50 ng) in HS buffer containing 0.15 m NaCl. Samples were then supplemented with [35S]cysteine (0.1 μm; 5 μCi) and 0.15 mm PLP in a final volume of 50 μl. After incubation at 30 °C for 15 min, proteins were precipitated with ice-cold ammonium sulfate (final 67%) and centrifuged to remove excess and unbound [35S]cysteine. Protein-bound radioactivity was determined by scintillation counting. Identical samples in which wild-type Nfs1 was replaced with the Nfs1 (K263A) mutant served as respective background controls. Background counts were subtracted, and data are from three separate experiments. B, Nfs1 (100 ng) was incubated at 30 °C for 15 min with increasing amounts of Yfh1 or Isu1Sup (1× = 50 ng) in HS buffer containing NaCl (0.15 m), [35S]cysteine (0.1 μm; 5 μCi) and PLP (0.15 mm) (first step). Nfs1 (K263A) served as negative control. Proteins were precipitated with ammonium sulfate and centrifuged. The protein pellets were solubilized with HS buffer containing 0.15 m NaCl and 0.15 mm PLP and incubated with Isd11 (100 ng) at 30 °C for 15 min (second step). Samples were analyzed by nonreducing SDS-PAGE and autoradiography.
