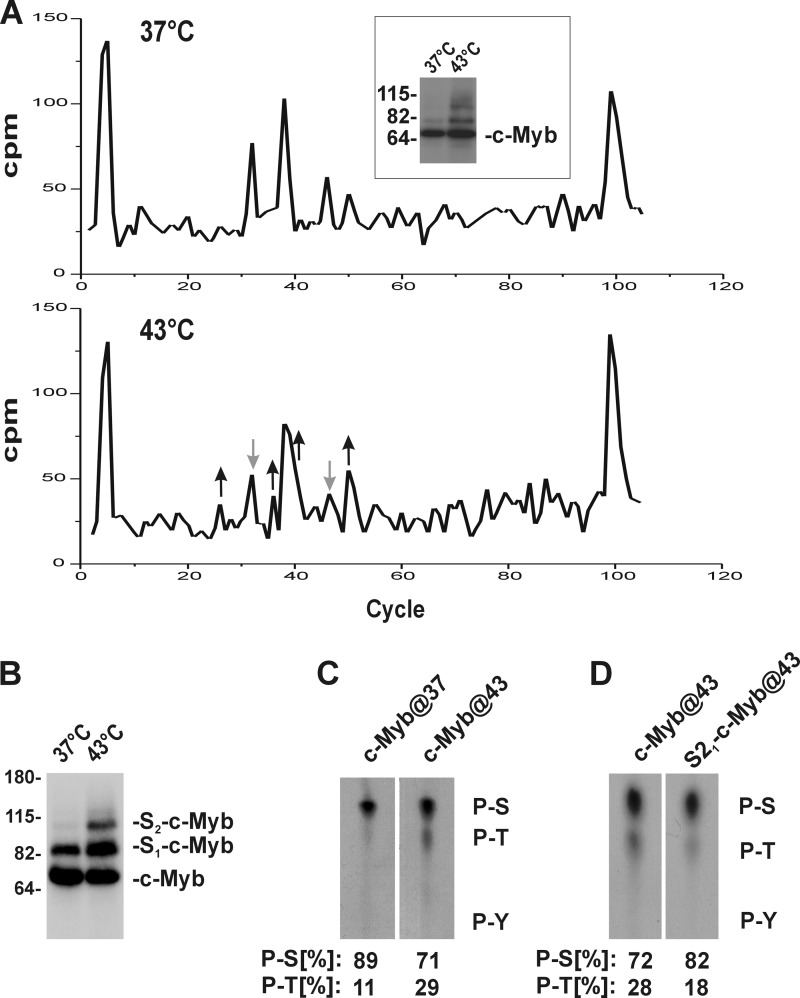
FIGURE 1.
Stress induces changes in phosphorylation of c-Myb. A, metabolically labeled c-Myb with [32P]orthophosphate was isolated from cell lysates prepared from unstressed (37 °C) or stressed cells (43 °C) by immunoprecipitation and separated by SDS-PAGE. Tryptic peptides from 32P-labeled c-Myb were separated by reverse phase HPLC. Black arrows pointing up and gray arrows pointing down mark fractions with increased and decreased intensity, respectively. The inset shows 32P-labeled c-Myb immunoprecipitated from COS-7 cells transfected with c-Myb construct and treated with heat stress as indicated. B, [32P]orthophosphate-labeled c-Myb immunoprecipitated from COS-7 cells transfected with constructs encoding c-Myb and HA-SUMO-2 and treated (43 °C) or untreated (37 °C) with hyperthermia was separated by SDS-PAGE, transferred to membrane, and directly exposed to x-ray film. Mobility of unmodified and SUMO-conjugated forms of c-Myb with one or two molecules of SUMO are labeled as c-Myb, S1-c-Myb, and S2-c-Myb, respectively. C, comparative phosphoamino acid analysis of the nonSUMOylated form of c-Myb isolated from cells untreated (c-Myb@37) or treated (c-Myb@43) with hyperthermia. D, comparative phosphoamino acid analysis of the nonSUMOylated (c-Myb@43) and the SUMOylated c-Myb (S21-c-Myb@43) isolated from cells treated with hyperthermia. The positions of the phosphoserine (P-S), phosphothreonine (P-T), and phosphotyrosine (P-Y) markers are visualized by ninhydrin staining and are indicated on the right. Quantitative analysis of radioactive signals was performed on a PhosphorImager 425 using ImageQuant software (Molecular Dynamics) and is shown as a percentage of phosphoserine and phosphothreonine; total phosphorylation of both phosphoserine and phosphothreonine was assigned to 100%.