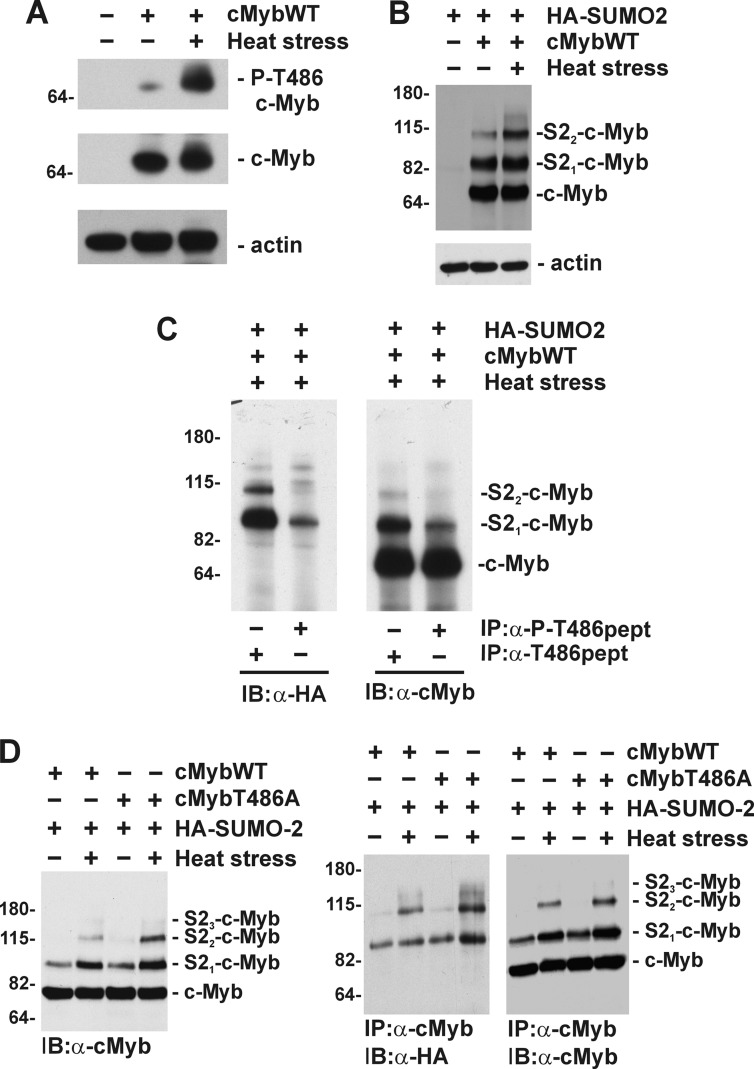
FIGURE 3.
Phosphorylation of Thr486 negatively regulates stress-induced SUMOylation of c-Myb. A, COS7 cells were transfected with a construct encoding c-MybWT and treated with hyperthermia as indicated. Immunoblotting results are of total cell lysates analyzed with rabbit polyclonal antibody specific for phosphorylated Thr486 (P-cMybT486, top panel), nonphosphorylated Thr486 in murine c-Myb (c-Myb, middle panel), or anti-actin antibody (bottom panel). B, cell lysates from COS7 cells transfected with construct encoding c-MybWT and HA-SUMO-2 and treated with hyperthermia were analyzed by immunoblotting with monoclonal anti-c-Myb (top panel), or anti-actin antibody (bottom panel). The mobility of unmodified (c-Myb) and SUMOylated forms (HAS21-cMyb and HAS22-cMyb) are marked. C, immunoprecipitation of c-Myb using rabbit polyclonal antibody specific for phosphorylated (α-P-T486pept) or nonphosphorylated Thr486 (α-T486pept) were analyzed by immunoblotting with monoclonal anti-HA (α-HA, left panel) or anti-c-Myb antibody (α-cMyb, right panel). D, COS7 cells transfected with HA-SUMO-2, c-MybWT, and c-MybT486A and treated with hyperthermia as indicated. Cell lysates analyzed by immunoblotting with monoclonal anti-c-Myb (α-cMyb, right panel). c-Myb was immunoprecipitated with rabbit anti-c-Myb polyclonal antibody and analyzed by immunoblotting with anti-HA (α-HA, middle panel) or anti-c-Myb (α-cMyb, right panel) monoclonal antibodies. The mobility of c-Myb or SUMOylated forms of c-Myb are indicated on the right, and molecular mass markers (in kDa) are indicated on the left. IP, immunoprecipitation; IB, immunoblotting.