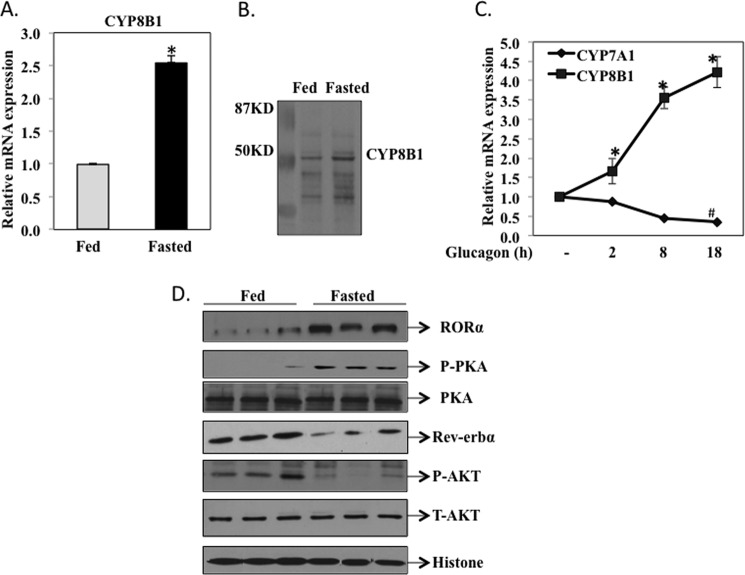
FIGURE 1.
Fasting induces CYP8B1 mRNA and RORα protein expression in hepatocytes. Male C57BL6J mice (12 weeks old) were fasted for 12 h from 8 a.m. (ZT2) to 8 p.m. (ZT14). A group of 4 mice was sacrificed at ZT14 as the fasted group, and another group (4) was re-fed a regular chow diet for 6 h and sacrificed at 2 am (ZT20) as the fed group. A, quantitative real-time PCR analysis of CYP8B1 mRNA expression in mouse liver. Relative CYP8B1 mRNA expression in fasted mouse liver was calculated with respect to fed mice. Results are expressed as the mean ± S.E.; n = 4; an asterisk indicates statistical significance, p < 0.05, fasted versus fed mice. B, Western immunoblot analysis of CYP8B1 protein in liver microsomes isolated from fasted and fed mice (pooled from n = 4). C, real-time PCR analysis of CYP8B1 and CYP7A1 mRNA expression in human primary hepatocytes (donors #1839, #1958, and #1959) treated with glucagon (100 ng/ml). mRNA samples were isolated from cells at the time points indicated. Results were expressed as mean ± S.E.; n = 3; * and # indicate statistical significance, p < 0.05, glucagon treated versus vehicle-treated cells. D, Western immunoblot analysis of RORα, Rev-erbα, PKA, p-PKA, AKT, and p-AKT in total liver extracts of fasted (16 h) and fed (6 h) mice. Each lane was loaded with liver extracts from different mice in each group. Histone 3 was used as a loading control.