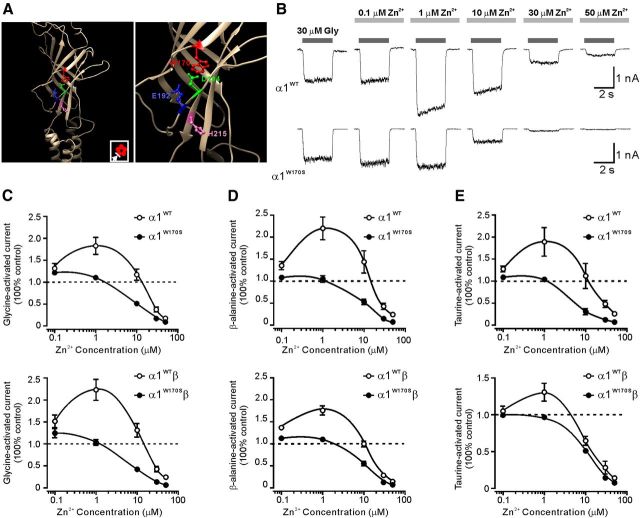
Figure 2.
W170S mutation impaired Zn2+-mediated potentiation and enhanced Zn2+-mediated inhibition of GlyR currents activated by different agonists. A, Left, The homology model of the GlyR α1 subunit based on the GluCl viewed from the outer face. Inset, Plan view of the GlyR pentamer. The arrow indicates the viewing angle. Right, The expanded illustration of the amino acid residues that affect Zn2+ potentiation. B, Representative traces represent biphasic modulation of Zn2+ on homomeric α1WT (top) or α1W170S (bottom) GlyR currents activated by glycine (EC10). C, Averaged Zn2+ concentration–response curves for the modulation of EC10 responses to glycine-activated currents in homomeric (top; α1WT, n = 7; α1W170S, n = 10) and heteromeric α1W170Sβ GlyRs (bottom; α1WT, n = 7; α1W170S, n = 10). D, Averaged Zn2+ concentration–response curves for the modulation of EC10 responses to β-alanine-activated currents in homomeric (top; α1WT, n = 5; α1W170S, n = 5) and heteromeric α1W170Sβ GlyRs (bottom; α1WT, n = 6; α1W170S, n = 5). E, Averaged Zn2+ concentration–response curves for the modulation of EC10 responses to taurine-activated currents in homomeric (top; α1WT, n = 5; α1W170S, n = 8) and heteromeric α1W170Sβ GlyRs (bottom; α1WT, n = 7; α1W170S, n = 5).