Abstract
Current fecal tests (occult blood, methylation, DNA mutations) target minute amounts of tumor products among a large amount of fecal material and thus have suboptimal performance. Our group has focused on exploiting field carcinogenesis as a modality to amplify the neoplastic signal. Specifically, we have demonstrated that endoscopically normal rectal brushings have striking nano-architectural alterations which are detectable utilizing a novel optical technique, partial wave spectroscopic microscopy (PWS). We therefore wished to translate this approach to a fecal assay.
We examined mucus layer fecal colonocytes (MLFCs) at preneoplastic and neoplastic time-points (confirmed with rat colonoscopy) in the azoxymethane (AOM)-treated rat model and conducted PWS analysis to derive the nano-architectural parameter, disorder strength (Ld). We confirmed these results with studies in a genetic model (the Pirc rat).
We demonstrated that MLFC appeared microscopically normal, consistent with field carcinogenesis. Ld was elevated at an early time point (5 weeks post-AOM injection, effect size = 0.40, p value = 0.024) and plateaued prior to adenoma formation (10 weeks post-AOM, effect size =0.66, p=0.001), with no dramatic increase once tumors developed. We replicated these data in the pre-neoplastic Pirc rat with an effect size in the MLFC that replicated the rectal brushings (increase versus age-matched controls of 62 versus 74%, respectively).
We provide the first demonstration of a biophotonics approach to fecal assay. Furthermore, targeting the nano-architectural changes of field carcinogenesis rather than the detection of tumor products may provide a novel paradigm for colorectal cancer screening.
Keywords: Colorectal cancer screening, biophotonics, nanocytology, fecal test, field carcinogenesis
INTRODUCTION
Despite a plethora of widely available tests, colorectal cancer (CRC) remains the second leading cause of malignancy-related mortality in the United States, underscoring the need for more effective population screening strategies(1). Typically, there is a trade-off between accuracy and patient acceptability/cost in screening tests. For instance, colonoscopy is the “gold standard” for accuracy but is plagued by patient compliance issues (attributable to discomfort, embarrassment, risk of complications, unpleasantness of colonic purge). Further complicating matters is the juxtaposition of the resource-intensive nature of colonoscopy or other imaging modalities (i.e. CT colography, capsule endoscopy) versus the remarkably low yield with screen-relevant neoplasia in the at-large population (well under 10%)(2). Therefore it is clear that “personalizing” risk analysis is essential for more accurate risk stratification, rather than simply designating the majority of the population 50 years and older as being “average risk”, which is the current state of the art(2).
In this regard, developing a pre-screen using a non-invasive, inexpensive approach would be of great value in determining which “average risk” populations are likely to harbor lesions and thus achieve a survival benefit from colonoscopy(3). One standard approach has been to use fecal assays which generally target tumor-related bleeding via fecal occult blood tests (guiaic or immunohistochemical) or tumor products (e.g. fecal DNA). While these tests have been documented to decrease fatalities from colorectal cancer, the insensitivity to advanced adenomas (and hence cancer prevention) has led them to be relegated to a second line test in some guidelines(4). Efforts to improve sensitivity using next-generation technologies including DNA mutation analysis, methylation etc. have been marginally successful (sensitivity for advanced adenomas ~50%)(5). Conceptually, fecal DNA assays may be limited by the proverbial “needle in a haystack” since human DNA only accounts for 0.01% of the DNA found in the stool(6). This is compounded further by the shedding of the entire intestinal epithelium every 3–7 days which means that only a small fraction of the human DNA would come from the neoplastic lesion.
Therefore, it is of paramount importance to amplify the neoplastic signal. One approach would be to exploit the diffuse alterations associated with field carcinogenesis (also known as field effect or field of injury)(7) thus targeting the much larger non-dysplastic mucosa(8). Field carcinogenesis is a well-established biological concept that provides the underpinning for several aspects of current clinical practice. For example, identification of an adenoma mandates assessment for concurrent (synchronous) lesions (full colonoscopy if a polyp is found on flexible sigmoidoscopy)(9) and future (metachronous) lesions (follow up colonoscopy at more frequent intervals given the high risk of recurrent lesions)(10). Since it is clear that adenoma appears to be a relatively insensitive marker for field carcinogenesis, there has been interest in assessing biomarkers that occur at an earlier stage (i.e. microscopically normal mucosa). Attention has focused on a variety of molecular markers (methylation, genomics, proteomics etc.)(11) that have been shown to be altered in the endoscopically-normal distal colonic mucosa, although the performance/practicality of these assessments presents difficulties in translating to clinical practice(8).
Our group has developed partial wave spectroscopic microscopy (PWS), an optical technology that evaluates the nano-architectural consequences of the subtle genetic/epigenetic changes in field carcinogenesis(12). Specifically, PWS is sensitivite to structures at 10–100 nm, thereby allowing quantification of the fundamental cellular “building blocks” (nucleosomes, ribosomes, macromolecular complexes etc.) that have been implicated in early carcinogenesis(12).
We have previously demonstrated that PWS analysis showed profound nanocytological changes (quantified by the parameter disorder strength (Ld)) from the microscopically normal rectal mucosal brushings in patients harboring neoplasia elsewhere in the colon(13). Furthermore, the magnitude of rectal Ld changes mirrored the CRC risk. However, for optimal clinical implementation, adaptation to a fecal test would be required. The major obstacle to date has been that the vast majority of fecal colonocytes are apoptotic and thus unsuitable for micro-architectural analysis. Recently, however, mucus layer fecal colonocytes have been isolated which appear to be morphologically well preserved (non-apoptotic)(14). We therefore wanted to determine whether PWS analysis of mucus layer fecal colonocytes could predict risk of CRC at pre-neoplastic time points (recapitulating field carcinogenesis). For these studies we used a well-validated model of CRC, a carcinogen model (the azoxymethane-treated rat) which was complemented by a genetic model, (the Pirc rat). (15)
MATERIALS AND METHODS
Animal Studies
All studies were performed under the auspices and supervision of the Institutional Animal Care and Use Committee of NorthShore University HealthSystem.
Azoxymethane (AOM)-treated rat
Male Fisher 344 Rats were obtained at 7–8 weeks of age and fed the AIN 76-A diet (Harlan Teklad, Madison, WI). Rodents were randomized with two weekly intraperitoneal injections of either AOM (15mg/kg of weight) or saline (Harlan Teklad, Madison, WI). We first wanted to determine if the premalignant colonocytes would manifest alterations in Ld. The AOM-treated rat has a well-defined time line with adenomas requiring ~15–20 weeks to develop and carcinomas ~35–40 weeks. We looked at the earliest time points (4 – 8 weeks post-AOM) by which time the non-specific (toxic) effects of the carcinogen have dissipated.(16,17)
Pirc (Polyposis in rat colon) Rat
Twenty Pirc rats were obtained from Taconic Farms (Germantown NY): These rats contain a germline mutation in the adenomatous polyposis coli (APC) tumor suppressor at codon 1137 leading to the development of multiple colonic neoplasms in ~3–4 months(15).
Rat Colonoscopy
Serial colonoscopic evaluation was performed via rigid rat colonoscope (Coloview™; Karl Storz, Culver city CA). Overnight fasted rats were sedated (with Isoflurane), secured in the supine position and a well lubricated colonoscopic probe introduced slowly via anus with gentle air insufflation. The tumor images were captured during the probe withdrawal utilizing the Image 1 camera system (Karl Storz).
Fecal Colonocyte Isolation
This was performed by modification of a protocol by White and colleagues(14). Fresh stool was collected within two hours of evacuation. Stool was processed from AOM and Saline groups as follows: An aliquot of stool was placed in a plastic bag and washed in PBS 1X. The sample was then agitated in 0.05% ammonium thioglycolate/PBS wash at a ratio of 2mL/gram of stool (Sigma Aldrich, St. Louis, MO) and pressed to liberate mucus from the outer fecal pellet. 5mL of thioglycolate wash was added to the sample and centrifuged at 1000 rpm for 5 min at 4°C. Samples were then decanted and re-suspended in CytoPreserv solution (Hologic, Bedford,MA) at a ratio of 5mL/gram of original stool. Samples were incubated at 4°C for one hour. Samples were then filtered through a 300uM WhirlPak Bag (Nasco, Fort Atkinson, WI) to remove large debris. Samples were further filtered through a 125uM mesh (Small Parts, Inc.) to collect the fecal mucus layer. Samples in the mesh were collected in 30mL of CytoPreserv solution and then centrifuged at 800 rpm for 5 min at 4°C, forming a thin mucus layer above the pellet. Mucus layer was removed and incubated in a combination of 1.5mM EDTA and 0.5M of N-acetyl L-cysteine (Sigma Aldrich, St. Louis, MO) for 10 minutes at 37°C. Samples were then added to CytoLyt solution (Hologic) to dilute mucolytic agents and fixed onto glass slides using ThinPrep 2000 (Hologic).
PWS Analysis
The PWS instrument used for this study has been previously described(12,18). PWS measures the disorder strength of intracellular architecture using the parameter (Ld). Ld = LCδn2, where δn2 is the variance of the spatial refractive index fluctuations. In short, for a given specimen, the PWS system generates a 3-D data-cube termed as the fluctuating part of the reflection coefficient where (x,y) refers to a specific pixel in the object plane and λ is the wavelength. For a given unstained cell, the spectral fluctuations are calculated in the wavelength range of 500 – 700 nm (above the system noise floor) by means of 1D mesoscopic light transport theory to obtain Ld. Thus, a map of disorder strength is obtained from each pixel (x,y). Using this 2-D map, for each cell the mean intracellular disorder strength (the average over x and y pixels) is obtained. The average for a group of cells (~ 20 – 30 cells for each time-point) is calculated and defined as the mean disorder strength per time-point. The Ld average and the standard error are depicted in all the histograms in this report. We note that slide-to-slide variability for each time-point was negligible (P-value > 0.10). All of the PWS analysis was performed by an investigator who was blinded to origin of cells/tissue (saline-treated versus AOM or wildtype versus Pirc rat).
Rectal Brushings
Rectal cells from the visually normal mucosa of the Pirc and age-matched wildtype rats were obtained with a cytology brush. The brush was gently applied to a microscope slide, fixed with 70% ethanol, air-dried and then subjected to PWS analysis.
5-bromo-3-deoxyuridine (BrdU) Pulse-Chase Experiments
To determine if isolated fecal colonocytes were obtained through epithelial abrasion from stool passage, rats were injected with 50 mg/kg of (BrdU) (Sigma Aldrich, St. Louis, MO) dissolved in sterile PBS. BrdU is a thymidine analog which is incorporated into proliferating cells. After incorporation, colonocytes migrate up the crypt in a time-dependent fashion(19). After 48 hrs fresh stool was collected and animals were euthanized. Four micron distal rat colon section and fecal colonocyte slides underwent BrdU immunostaining with a biotinylated mouse anti-BrdU antibody (Invitrogen, Carlsbad,CA.) as previously described(20).
Statistical Methods
All statistical analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA). A standard two tailed t-test (assuming unequal variances) with p values ≤0.05 considered statistically where μ1 & μ2 are the means for the AOM-treated or Pirc models and control groups, respectively, and σ1 & σ2 are the corresponding standard deviations. We used this effect size definition to take into account the slide-to-slide variability and to robustly measure the statistical significance of the average Ld difference, i.e., for control and carcinogen-treated or Pirc rat.
RESULTS
Experimental Models
We utilized both carcinogen and genetic experimental models in these studies given the time-frame for neoplastic development was well characterized and the animals could be followed longitudinally with fecal sampling at various time points. We utilized a rigid colonoscope to determine the tumor-bearing status in the distal 80% of the colon where the vast majority of tumors are located.
The ability to visualize the colon in vivo was excellent. The age-matched control (saline injected) rat was tumor free (Fig. 1a) whereas Fig 1b shows representative images with the AOM-treated rat indicating distinct tumors at 24 weeks. Similarly, the age-matched wildtype rats were tumor free (Fig 1c) whereas the PIRC rat colon showed the characteristic multiple adenomas (polyposis) (Fig 1d).
Figure 1. Representative colonoscopic images from animal models of experimental colon carcinogenesis.
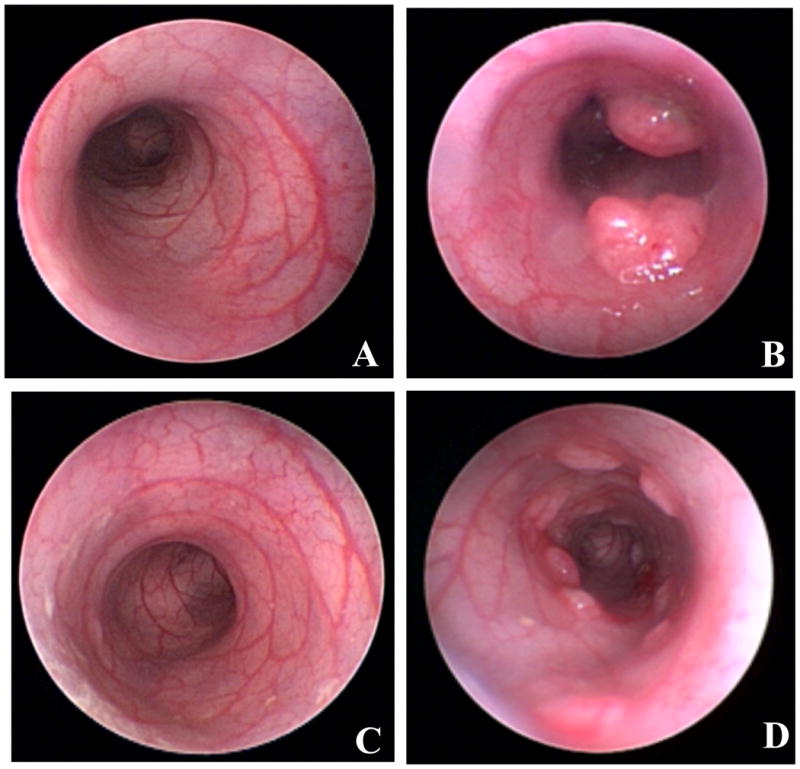
For these longitudinal studies, rat colonoscopy was performed to evaluate tumorigenesis status of animals at the time point of fecal sampling of AOM-treated rat without and with tumor.
A) Twenty-four week old Fisher 344 controls (saline treated) without tumors.
B) Twenty-four week old Azoxymethane (AOM) treated Fisher 344 rats with adenomas.
C) APC Wildtype rat colon (14 weeks old)—no tumor noted.
D) Pirc rat -14 weeks old with evidence of polyposis with numerous adenomas detectable.
Mucus Layer Fecal Colonocytes: Isolation and Characterization
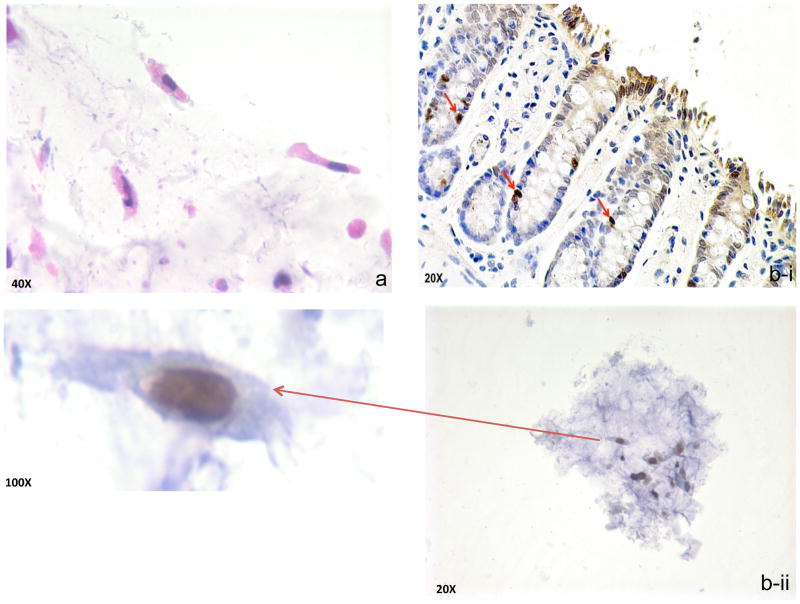
Stool was recovered immediately prior to rat colonoscopy. With regard to fecal colonocytes, our yield was ~15 cells/g of stool. Since our previous works indicated that PWS analysis of field carcinogenesis necessitates ~30–50 cells, we utilized ~2–4g of stool aliquots for this analysis(13,21). Review by pathologist (S.E.C.) confirmed that the majority of cells (~80%) were morphologically preserved (non-apoptotic) colonocytes (Fig 2a) with the remainder being squamous (presumably anal in origin) or scattered inflammatory cells (macrophages etc). To further support the notion that the colonocytes were abraded by stool bolus rather than sloughed by apoptosis, we wanted to analyze the location of these cells in regard to the crypt. We reasoned that since apoptotic cells are sloughed from the tip of the crypt, if we were able to demonstrate that some of the cells were at the base of the crypt, this would strongly argue against apoptosis(22). To label the cells, we injected animals with 5-bromo-3-deoxyuridine (BrdU) which is incorporated into proliferating cells which reside in the base of the crypt. Typically, these labeled cells migrate towards the top of the crypt as they mature(~5–7 days)(19). We confirmed 2 days post-injection (fig 2b-i) that all the labeled colonocytes (specifically nuclei since BrdU is incorporated into DNA) were confined to the bottom half of the crypt (there was some nonspecific cytoplasmic blush towards the luminal surface). Importantly, we were able to note in the stool recovered that a small proportion (~3–5%) of the mucus layer fecal colonocytes had evidence of BrdU uptake, suggesting that they were not from the apoptotic areas (tip of the crypt) (figure 2b-ii).
Figure 2. Mucus Layer Fecal Colonocyte isolation and characterization.
a. Representative fecal colonocytes demonstrating well-reserved columnar
- Section of colon stained with BrdU (20X magnification)—14 week old AOM-treated rats 48 hours post injection where positive nuclei are confined to the bottom half of the crypts. Since BrdU is incorporated in the nucleus, the cytoplasmic blush at the luminal surface is artifactual.
- Mucus layer fecal colonocytes obtained from these same animals immediately prior to tissue biopsy.
PWS analysis of fecal colonocytes from AOM-treated rat model
First, we investigated the ability of PWS to differentiate microscopically normal fecal colonocytes isolated from the pre-dysplastic AOM-treated rats (n=4) versus age-matched saline (n=4). We employed the AOM-treated rat because it is well validated (the leading model of experimental colonic neoplasia over the last several decades) and the neoplastic timeline is well established (i.e. aberrant crypt foci, adenomas and carcinomas ~5, ~20 and ~35–40 weeks, respectively)(16,17). Furthermore, this model allows ready longitudinal analysis for tumors via rat colonoscopy. No time points prior to 2 weeks were evaluated since it has been demonstrated that there may be non-specific carcinogen effects, however this dissipates within 10 days after carcinogen treatment(16).
As previously noted, fecal colonocytes appeared microscopically identical suggesting that structures at length scales of above ~200–500 nm (diffraction limit of light) were largely unchanged (Fig 3a). However, at smaller length scales of PWS analysis (>10–20 nm), there were marked differences. Indeed, when a disorder strength (Ld) pseudo-color map was superimposed on the images, there appeared to be a marked increase in Ld in the fecal colonocytes from AOM-treated animals (denoted by red-color coded regions) when compared to the age-matched saline treated controls. (Fig 3a) The Ld appeared to be altered between AOM and saline throughout the cell, consonant with our previous work which had noted that differential signals occurred both in the nucleus (high order chromatin) and cytoplasm (driven at least partially by cytoskeletal alterations).(23)
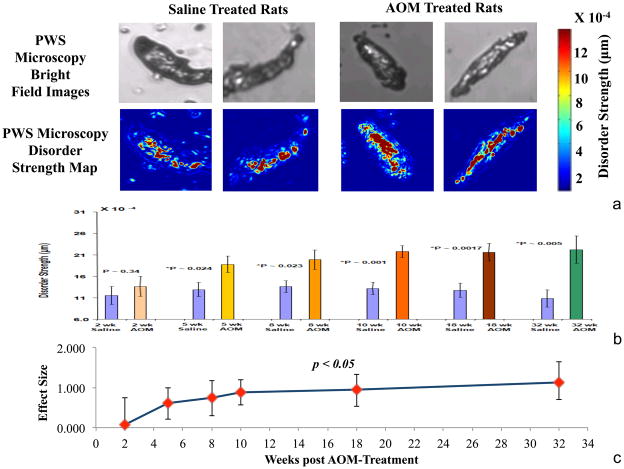
Figure 3. PWS analysis of AOM-treated rat.
a. PWS analysis of fecal colonocytes: Using the bright field capability of the PWS microscope, images of unstained fecal colonocytes from both AOM treated (at pre-tumorigenic timepoints) and control animals were taken and appeared identical. However, using Ld pseudo-color map was markedly different with higher Ld areas in the AOM-treated rat being noted in both the nucleus and cytosol.
b. Ld Quantification: Since the saline treated animal fecal colonocytes Ld did not change over time (see supplementary figure) all time-points were collectively used as comparator. With regards to AOM-treatment there was an early rapid stepwise induction in mean cellular Ld with significant differences noted and 5 weeks and plateauing ~10 weeks post carcinogen.
c. Effect Size: In order to gain insights into the diagnostic performance, we examined “effect size” of mean cellular Ld (versus saline control) and was plotted with 95% confidence intervals. Similar to the absolute differences, the effect size was significantly increased at 5 weeks and the magnitude appeared to plateau ~10 weeks post carcinogen treatment.
We then quantified the mean fecal colonocyte Ld at various time points during carcinogenesis (Fig 3b & 3c). When compared to age-matched controls, there was no difference in disorder strength (ΔLd) at 2 weeks post-AOM treatment (effect size = 0.052, p value = 0.82) while all other time-points manifested a significant difference. The standard deviations were large presumably related to the somewhat skewed Ld distribution in both the saline and AOM-treated colonocytes. For instance, ΔLd increased significantly (effect size = 0.40, p value = 0.024) at 5 weeks after the AOM treatment. The differential became more pronounced at week 8 (Effect-size = 0.47, P-value = 0.023), and was maximal after week 10 (effect size = 0.66, p value = 0.001), and 18-week (effect size = 0.70, p value = 0.0017) post-AOM treatment. Importantly, previous results have suggested that this time-point may best replicate human field carcinogenesis(16).
It needs to be emphasized that these alterations in nanoscale structure in carcinogenesis reflect field changes and occurred prior to tumorigenesis, and thus were not confounded by tumor-related fecal colonocytes. While the focus of our studies was mainly on premalignant time-points, the 32-week animals did have evidence of tumors. The salient finding at this time-point was that while the fecal colonocytes still manifested a highly diagnostic elevation in Ld, this was equivalent to earlier times (weeks 15 and 20), suggesting that the tumor per se was not adding perceivably to Ld from the mucus layer fecal colonocytes.
Results for Pirc Rats Rectal Brushing
We next wanted to demonstrate that the fecal colonocyte approach was not model specific. Therefore, we selected a genetic model, the Pirc rat (polyposis in rat colon) to complement the carcinogen rat study. The advantage of this model is that given its germline mutation in the adenomatous polyposis coli (APC) tumor suppressor gene, it recapitulates the genetic initiation of most sporadic human colorectal cancers. To demonstrate that PWS was effective at identifying field carcinogenesis in this model, we used rectal brushings at a time-point prior to adenoma development (8 weeks of age, pre-adenoma status confirmed by rat colonoscopy). These rectal brushings (n = 5 wildtype versus n= 7 Pirc) were applied to a glass slide and then underwent PWS analysis on the colonocytes. As indicated in Fig. 4a, there was a 74% increase in ΔLd (Effect size =0.47, p value=0.0005) between the Pirc and age-matched APC wildtype rats. When we analyzed mucus layer fecal colonocytes (Fig. 4b) from corresponding animals (8 weeks of age) we found that the ΔLd was increased 62% (Effect size = 0.39, p value = 0.03). The absolute Ld values were somewhat altered between brushing and fecal colonocytes but may relate to the different fixation techniques. Importantly, the similarities between the rectal brushing and fecal colonocyte effect-size data provide further corroboration that mucus layer fecal colonocytes are actually being obtained from the normal (non-apoptotic) epithelium. Hence, the effect observed by PWS analysis performed on the mucus layer fecal colonocytes is not limited by the animal model but could be a universal phenomenon.
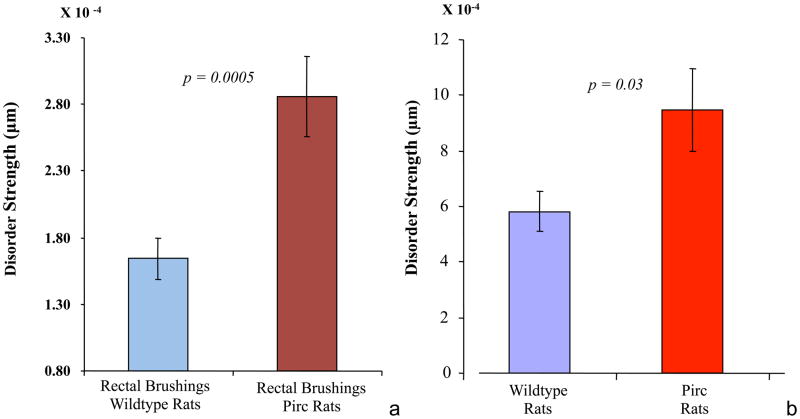
Figure 4. PWS analysis of Pirc rats.
a. Rectal brushings- PWS-measured Ld was significantly higher (74% increase, P-value=0.0005) for isolated rectal colonocytes obtained from 8-week old Pirc rats (n = 7 animals) compared to age-matched APC wildtype rats (n = 5 animals).
b. Fecal colonocytes: PWS results from fecal colonocytes of Pirc rats--Ld was significantly higher (62% increase, P-value=0.03) for isolated fecal colonocytes obtained from 8-week old Pirc rats compared to age-matched APC wild-type rats.
DISCUSSION
We report herein that PWS analysis of mucus layer fecal colonocytes was able to detect the early nano-architectural signatures of field carcinogenesis and hence identify risk of colonic neoplasia. We demonstrate this in two well-validated models: a carcinogen and a genetic model of CRC. Importantly, we focused on premalignant mucosa (colonoscopically confirmed), thus replicating the potential clinical applications. This is the first demonstration that optical techniques can be used as fecal assays. Furthermore, it shows the power of PWS to practically detect nano-architectural aberrations in cytologically normal epithelial cells.
The approach of identifying field carcinogenesis rather than tumor products/tumor-related bleeding provides a different paradigm for fecal assays. Colon field carcinogenesis can be identified by morphological characteristics (adenomas or aberrant crypt foci) or biomarkers from the microscopically normal mucosa such as cellular (apoptosis/proliferation)(24) and molecular (genomic, proteomic, microRNA, methylation etc) markers (25–28). However, all of these require instrumenting the colon, presenting a hurdle to patient uptake. Stool studies can overcome these obstacles and several stool biomarkers have been shown to be altered in field carcinogenesis (vimentin methylation or microRNAs such as hsa-miR-342 or miR 137)(29,30).
Nano-architectural biomarkers are particularly attractive because they may represent a common pathway for a myriad of pre-neoplastic genetic/epigenetic events. Our previous work has indicated that PWS is exquisitely sensitive to subtle alterations in expression of tumor suppressor genes and proto-oncogenes, with Ld correlating well with a more malignant phenotype in microscopically identical cells(12). We also noted that in the MIN mouse (the murine equivalent of the Pirc rat), the biophotonic analysis of brushings at a pre-dyplastic time-point accurately discriminated between animals with and without the APC mutation(31). We have demonstrated in human studies that brushings of the endoscopically normal mucosa showed a progressive induction of Ld in patients with non-advanced adenomas, advanced adenomas and carcinomas (~ 125%, ~ 225% and ~ 400% of control, respectively)(13). Thus, nanocytology represents a powerful modality for field effect identification.
Translation of PWS to fecal colonocyte analysis has to overcome an obstacle in that most fecal colonocytes shed via apoptotic and are thus unlikely to retain informative ultrastructural data. Several lines of evidence support our thesis that mucus layer fecal colonocyte are structurally normal colonocytes and thus can represent field carcinogenesis. For instance, our data (consistent with others) have shown that they were generally morphologically preserved(32). Moreover, the finding that some mucus layer fecal colonocytes showed BrdU incorporation (at a time-point when tissue BrdU positive cells was confined to the bottom half of the crypt) suggests non-apoptotic mechanisms for extrusion of these cells in the colonic lumen. This provides strong support for the notion that these cells are normal epitheliums which are abraded by the passage of a formed stool bolus. In this regard, it is interesting to note that the effect size was equivalent between the rectal brushings and fecal colonocytes, further corroborating the abrasion concept.
The ability to sense the nanoscale correlates of field carcinogenesis is a testament to the power of PWS for sensing sub-diffractional length scales and cellular structure. Because optical refractive index is a linear function of the local density of intracellular solids (proteins, lipids, DNA and RNA), the spectrum of a scattering signal contains information about spatial variations of density at length scales that are well below the wavelength. This is the main principle of PWS, which is capable of extracting one-dimensionally-propagating waves from different parts of a scattering particle such as a cell. Our previous studies have shown that in realistic experimental conditions the limit of PWS sensitivity to structures is under 20 nm(33). Thus, PWS opens up the realm of cellular nanostructure for quantification.
The identity of the structures that lead to the altered Ld in fecal colonocytes has yet to be elucidated. Our previous reports indicate that the signal can emanate from both the nucleus and cytoplasm. However, there are myriad candidate structures at this length scale that are integral to colon carcinogenesis including mitochondria/ribosomes and high order chromatin(34, 23).
In addition to the technological breakthrough of PWS, another innovation lies in clinical application. Most studies on novel optical technologies have largely focused on the optical biopsy—identifying the histology of lesions in situ which can be quite important in the application of endoscopy(35). Our focus has been on risk stratification via field carcinogenesis detection in a variety of organs including colon, lung, pancreas, ovary and esophagus(18,21,34). Our previous work showed that PWS nanocytology from the rectum was able to predict both concurrent and future neoplasia in patients via brushings of the endoscopically normal rectal mucosa 13. The clinical imperative for CRC risk stratification is that the vast majority of screening colonoscopies (>90%) are unproductive from a cancer prevention perspective, while much of the population lacks access to these finite endoscopic resources. The current strategy of grouping all patients ≥50 years of age without a personal and family history of colonic neoplasia as “average risk” is biologically naïve and leads to inefficiency. Attempts to stratify based on standard CRC risk factors (family history, diet, obesity, tobacco, etc.) yielded an improvement but still offer fairly modest predictive ability (AUROC of NIH risk score for CRC is 0.60)(36). We speculate that fecal PWS may have the requisite clinical performance based on the power of the technology and because field carcinogenesis is impacted by both genetic and exogenous factors.
Furthermore, a two-step process (non-invasive pre-screen, with positive results being offered the more definitive colonoscopy) is the basis of current approaches such as flexible sigmoidoscopy, CT colography, and the widely used fecal approaches such as the fecal immunohistochemical test (FIT). Fecal tests are particularly attractive given their low cost and minimal intrusiveness. It needs to be highlighted that improved compliance engendered by fecal tests can ameliorate differences in accuracy as demonstrated by the equivalent CRC yield of FIT and colonoscopy in a recent study. Thus, since this early report appears to suggest that field carcinogenesis detection with fecal PWS has considerable promise, the future potential public health implications for this approach may be substantial.
There are several limitations to this work that need to be acknowledged. First, it exclusively involves animal models such that the translatability to humans needs to be confirmed. On the other hand, the demonstration in both a well-validated carcinogen and a genetic model ameliorates this concern. Furthermore, we have previously published that PWS is highly accurate at detecting field carcinogenesis in humans via rectal brushings(13). Secondly, the issue of whether high-quality mucus layer fecal colonocytes will be recoverable in humans needs to be addressed. While our studies were exclusively on rats, White and colleagues have reported copious colonocytes recovered in human feces by a similar protocol(14). Thirdly, the effect of concurrent neoplasia was not rigorously explored although the thrust of this approach is to evaluate risk and, if anything, one would expect either an enhanced or unaffected diagnostic performance (consistent with our 32-week AOM rat data).
In conclusion our report provides the proof of principle that fecal colonocyte analysis can be utilized for field carcinogenesis detection which can be identified using a powerful new biophotonics approach, PWS. This is the first report on using biophotonics for fecal colonocyte analysis and thus the uncoupling of GI tract optical applications from endoscopy. This approach may herald a change in fecal tests from simple detection of minute quantities of neoplastic products in the fecal stream to the more amplified field carcinogenesis signal, thus enabling the prediction of both current and future risk. From a clinical perspective, fecal PWS analysis may serve as a pre-screen to identify both patients likely to benefit and, equally importantly, those who can safely eschew colonoscopy. This approach could be a paradigm shift, allowing for the personalization of colorectal cancer screening.
Supplementary Material
Acknowledgments
Financial Support/Funding: Supported by grants from the NIH U01CA111257, R01CA156186, R01 CA165309, R01CA128641, R42CA168055
Footnotes
Competing Interests: Drs. Roy, Backman and Subramanian are co-founders and shareholders in Nanocytomics LLC. All other authors do not have competing interests.
Author Contributions: HKR and VB developed the concept and designed the study and provided overall supervision. MDC, DPK, AKT and RKW developed fecal assays. DPM and HS performed the PWS analysis. SEC was the pathologist. Manuscript was written by HKR, VB and DPM with contribution/approval of co-authors.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Roy HK, Bianchi LK. Colorectal cancer risk: black, white, or shades of gray? Jama. 2008;300:1459–61. doi: 10.1001/jama.300.12.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248–56. doi: 10.1053/j.gastro.2011.10.031. quiz e25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlquist DA. Molecular detection of colorectal neoplasia. Gastroenterology. 2010;138:2127–39. doi: 10.1053/j.gastro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Steiling K, Ryan J, Brody JS, Spira A. The field of tissue injury in the lung and airway. Cancer Prev Res (Phila) 2008;1:396–403. doi: 10.1158/1940-6207.CAPR-08-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein C, Bernstein H, Payne CM, Dvorak K, Garewal H. Field defects in progression to gastrointestinal tract cancers. Cancer Lett. 2008;260:1–10. doi: 10.1016/j.canlet.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein C, Nfonsam V, Prasad AR, Bernstein H. Epigenetic field defects in progression to cancer. World journal of gastrointestinal oncology. 2013;5:43–9. doi: 10.4251/wjgo.v5.i3.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian H, Pradhan P, Liu Y, Capoglu I, Li X, Rogers J, et al. Optical Methodology for Detecting Histologically Unapparent Nanoscale Consequences of Genetic Alterations in Biological Cells. Proceedings of the National Academy of Sciences. 2008;105:20124–29. doi: 10.1073/pnas.0804723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damania D, Roy HK, Subramanian H, Weinberg DS, Rex DK, Goldberg MJ, et al. Nanocytology of rectal colonocytes to assess risk of colon cancer based on field cancerization. Cancer Res. 2012;72:2720–7. doi: 10.1158/0008-5472.CAN-11-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White V, Scarpini C, Barbosa-Morais NL, Ikelle E, Carter S, Laskey RA, et al. Isolation of stool-derived mucus provides a high yield of colonocytes suitable for early detection of colorectal carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2006–13. doi: 10.1158/1055-9965.EPI-08-1145. [DOI] [PubMed] [Google Scholar]
- 15.Amos-Landgraf JM, Kwong LN, Kendziorski CM, Reichelderfer M, Torrealba J, Weichert J, et al. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci U S A. 2007;104:4036–41. doi: 10.1073/pnas.0611690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy HK, Liu Y, Wali RK, Kim YL, Kromine AK, Goldberg MJ, et al. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology. 2004;126:1071–81. doi: 10.1053/j.gastro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Wali RK, Roy HK, Kim YL, Liu Y, Koetsier JL, Kunte DP, et al. Increased Microvascular Blood Content is an Early Event in Colon Carcinogenesis. Gut. 2005;54:654–60. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian H, Roy HK, Pradhan P, Goldberg MJ, Muldoon J, Brand RE, et al. Nanoscale cellular changes in field carcinogenesis detected by partial wave spectroscopy. Cancer Res. 2009;69:5357–63. doi: 10.1158/0008-5472.CAN-08-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy HK, Kunte DP, Koetsier JL, Hart J, Kim YL, Liu Y, et al. Chemoprevention of colon carcinogenesis by polyethylene glycol: suppression of epithelial proliferation via modulation of SNAIL/beta-catenin signaling. Mol Cancer Ther. 2006;5:2060–9. doi: 10.1158/1535-7163.MCT-06-0054. [DOI] [PubMed] [Google Scholar]
- 20.Kunte DP, Wali RK, Koetsier JL, Roy HK. Antiproliferative effect of sulindac in colonic neoplasia prevention: role of COOH-terminal Src kinase. Mol Cancer Ther. 2008;7:1797–806. doi: 10.1158/1535-7163.MCT-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy HK, Subramanian H, Damania D, Hensing TA, Rom WN, Pass HI, et al. Optical detection of buccal epithelial nanoarchitectural alterations in patients harboring lung cancer: implications for screening. Cancer Res. 2010;70:7748–54. doi: 10.1158/0008-5472.CAN-10-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loktionov A. Cell exfoliation in the human colon: myth, reality and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281–9. doi: 10.1002/ijc.22647. [DOI] [PubMed] [Google Scholar]
- 23.Damania D, Subramanian H, Tiwari AK, Stypula Y, Kunte D, Pradhan P, et al. Role of cytoskeleton in controlling the disorder strength of cellular nanoscale architecture. Biophys J. 2010;99:989–96. doi: 10.1016/j.bpj.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anti M, Armuzzi A, Morini S, Iascone E, Pignataro G, Coco C, et al. Severe imbalance of cell proliferation and apoptosis in the left colon and in the rectosigmoid tract in subjects with a history of large adenomas. Gut. 2001;48:238–46. doi: 10.1136/gut.48.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao CY, Moore DH, Chiu YS, Wong P, Bennington JL, Smith AP, et al. Altered gene expression in normal colonic mucosa of individuals with polyps of the colon. Dis Colon Rectum. 2005;48:2329–35. doi: 10.1007/s10350-005-0153-2. [DOI] [PubMed] [Google Scholar]
- 26.Polley AC, Mulholland F, Pin C, Williams EA, Bradburn DM, Mills SJ, et al. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006;66:6553–62. doi: 10.1158/0008-5472.CAN-06-0534. [DOI] [PubMed] [Google Scholar]
- 27.Balaguer F, Link A, Lozano JJ, Cuatrecasas M, Nagasaka T, Boland CR, et al. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–18. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paun BC, Kukuruga D, Jin Z, Mori Y, Cheng Y, Duncan M, et al. Relation between normal rectal methylation, smoking status, and the presence or absence of colorectal adenomas. Cancer. 2010;116:4495–501. doi: 10.1002/cncr.25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–32. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 30.Grady WM, Parkin RK, Mitchell PS, Lee JH, Kim YH, Tsuchiya KD, et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene. 2008;27:3880–8. doi: 10.1038/onc.2008.10. [DOI] [PubMed] [Google Scholar]
- 31.Roy HK, Kim YL, Wali RK, Liu Y, Koetsier J, Kunte DP, et al. Spectral markers in preneoplastic intestinal mucosa: An accurate predictor of tumor risk in the MIN mouse. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1639–45. doi: 10.1158/1055-9965.EPI-04-0837. [DOI] [PubMed] [Google Scholar]
- 32.Kunte D, DelaCruz M, Wali RK, Menon A, Stypula Y, Patel A, et al. Dysregulation of MicroRNAs in Colonic Field Carcinogenesis: Implications for Screening. PLOS One. 2012 doi: 10.1371/journal.pone.0045591. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian H, Pradhan P, Liu Y, Capoglu IR, Rogers JD, Roy HK, et al. Partial-wave microscopic spectroscopy detects subwavelength refractive index fluctuations: an application to cancer diagnosis. Opt Lett. 2009;34:518–20. doi: 10.1364/ol.34.000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backman V, Roy HK. Light-scattering technologies for field carcinogenesis detection: a modality for endoscopic prescreening. Gastroenterology. 2011;140:35–41. doi: 10.1053/j.gastro.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy HK, Goldberg MJ, Bajaj S, Backman V. Colonoscopy and optical biopsy: bridging technological advances to clinical practice. Gastroenterology. 2011;140:1863–7. doi: 10.1053/j.gastro.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman AN, Slattery ML, Ballard-Barbash R, Willis G, Cann BJ, Pee D, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686–93. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.