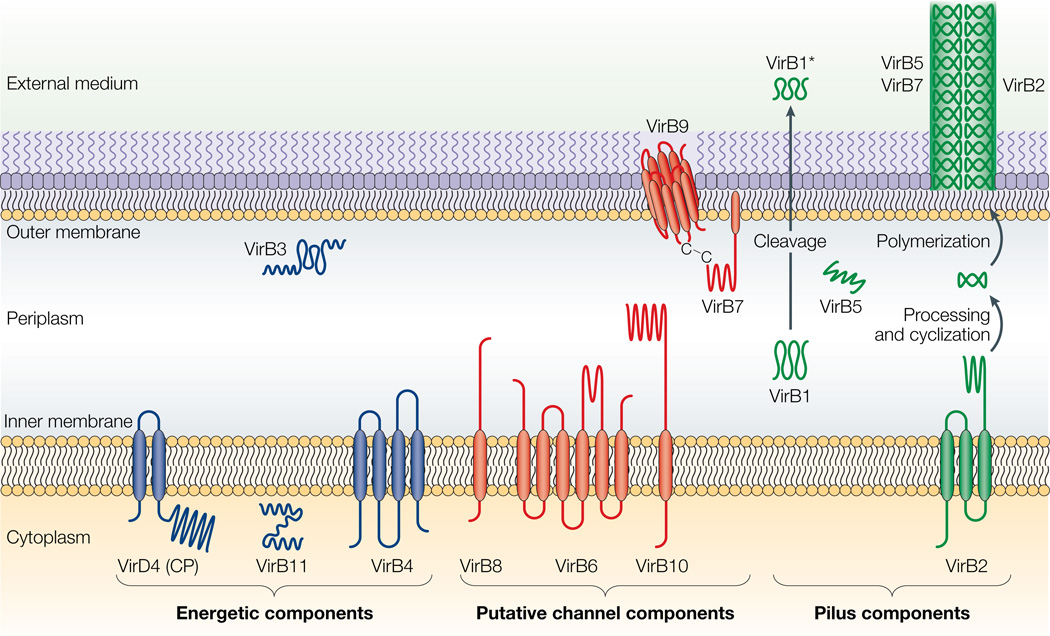
Figure 2. Topologies of the VirB/D4 subunits of the A. tumefaciens type IV secretion (T4S) system.
The coupling protein (CP) VirD4 and the mating-pore-formation components (VirB1–VirB11) are represented according to their proposed functions: energetic (blue), channel (red) or pilus (green) components. Several proteins are post-translationally modified in the periplasm. Signal sequences of VirB1, VirB2, VirB5, VirB7 and VirB9 are cleaved by signal peptidases. VirB1 is processed to form VirB1*, which is exported across the outer membrane. VirB2 undergoes a novel head-to-tail cyclization reaction, and polymerizes as the T-pilus. VirB7 is modified as a lipoprotein that associates with the T-pilus and also forms an intermolecular disulphide crosslink with VirB9, a possible secretin113. The VirB and VirD4 proteins are postulated to assemble as a supramolecular structure composed of a transenvelope channel and an extracellular pilus.