Abstract
The efficacy of antipsychotics in the treatment of schizophrenia depends on their ability to block dopamine (DA) D2 receptors. D2 receptor excitatory mediation of glutamatergic receptors has been implicated in in vivo studies. However, D2 receptor enhancement of glutamatergic transmission has rarely been reported in slice recordings. Instead, D2 receptor depression of both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartate (NMDA) action was obtained in previous slice studies. To obtain insight into this paradox, we examined DA's actions on synaptic responses of layer V pyramidal cells to minimal extracellular stimulation in layer III of ferret prefrontal cortical slices under NMDA and γ-aminobutyric acid type A blockade. This experimental design models the proposed hypofunction of NMDA receptor and γ-aminobutyric acid type A deficiency in schizophrenia. We found that DA and D2 receptor agonists promoted burst firing in a subset of pyramidal cells, which was reversed by haloperidol, a D2 antagonist and a D3 agonist, compounds having antipsychotic efficacy. In contrast, a D4 antagonist, which has not proven clinically effective, was not effective in blocking DA-promoted bursts. These results revealed excitatory effects of DA mediated mainly via D2 receptors, potentially providing a cellular mechanism for the D2 antagonism in treating schizophrenia.
Dopamine (DA) has been implicated in many diseases such as Parkinson's disease, Huntington's disease, and schizophrenia. Its effects are mediated through two major classes of DA receptors: the D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptors. The efficacy of typical antipsychotic drugs such as haloperidol is correlated with their affinities for the D2 class of receptor sites (1, 2), consistent with a hypothesis that D2 receptor signaling is elevated in schizophrenia. D2 receptor excitatory mediation of glutamatergic receptors has been implicated in in vivo studies (3, 4). However, it is puzzling that studies in cortical slices have generally revealed depressant rather than enhancing effects of D2 receptors on both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA)-mediated activities (5-8).
Considering the variable effects of DA on overall glutamatergic transmission (5, 7-10), in the present study we recorded the synaptic responses of neurons under NMDA and γ-aminobutyric acid type A (GABAA) receptor blockade to pharmacologically isolate the action of non-NMDA receptors on prefrontal neurons. This experimental design models the proposed hypofunction of NMDA receptor (11-14) and GABAA deficiency (15, 16) as pathophysiological mechanisms in schizophrenia. Single and dual whole-cell patch clamp recordings were carried out in the medial prefrontal cortical (mPFC) slices, an area of the brain implicated in schizophrenia (17) DA-promoted bursting and DA-enhanced non-NMDA EPSPs were elicited in a subset of layer V pyramidal cells (PCs) by extracellularly stimulating layer III with minimal stimulation strength.
Materials and Methods
Slice Preparation and Electrophysiological Recording. Prefrontal cortical slices were prepared from young ferrets (6-9 weeks old) following the experimental procedure used in our laboratory (18). The extracellular solution (artificial cerebrospinal fluid, ACSF) contained 125 mM NaCl, 2.5 mM KCl, 10 mM dextrose, 25 mM NaHCO3, 1.25 mM NaH2PO4, 1.5 mM CaCl2, and 1.5 mM MgCl2. Neurons from mPFC were identified by using infrared differential interference contrast (IR-DIC) video microscopy with an upright microscope (BX50WI, fitted with a ×40 W/0.80 numerical aperture objective; Olympus) according to pyramidal shape somata and thick primary apical dendrites typical for pyramidal neurons, and later verified by observation of stained neurons under light microscope (LM) and by 3D-computer reconstruction of sample neurons. Dual whole-cell patch-clamp recordings (6-10 MΩ pipette resistance) were carried out on layer V PCs with biocytin-loaded (0.2%) pipettes containing 138 mM K-MeSO4, 2 mM KCl, 2 mM MgCl2, 10 mM Hepes, 0.03 mM EGTA, 4 mM Na2-ATP, and 0.3 mM GTP (pH 7.3). To evoke excitatory synaptic potentials (EPSP), glass electrodes (2-3 MΩ pipette resistance) filled with ACSF were placed in layer III at a vertical distance of 250-300 μm from the recorded cell body. Stimulation pulses (duration, 1 ms) were controlled by a stimulus isolator (A360, World Precision Instruments, Sarasota, FL) and triggered by computer. The stimulation strength (3-10 μA) was determined once a minimal stable EPSP (amplitude, usually 1-8 mV) was recorded. The onset latency of EPSPs was consistent, and the rising time of the EPSPs was 3.1 ± 0.03 ms (mean ± SE, n = 60). Only neurons with resting membrane potentials of -50 mV or more negative were used for the recordings. Membrane potential of recorded PCs was clamped at -70 mV except where noted. Signals were amplified by using Axopatch-200B amplifiers (Axon Instruments). Recordings were sampled at 4 KHz, and low-pass prefiltered (4-8 pole Bessel) by using the program pulse (WaveMetrics), digitized by an ITC-18 interface (Instrutech, Port Washington, NY). The measurement and control of noise, failures, amplitudes of EPSPs, rise times of currents (RT), series resistance, and whole cell capacitance of voltage clamp recordings followed the published procedures (19).
Pharmacological Treatments. NMDA receptors and GABAA receptors were blocked in all experiments by bath application of 50 μM 2-amino-5-phosphopentanoic acid (AP-5) and 5 μM (-)bicuculline methiodide, respectively. DA (10-25 μM) was bath applied with 10 μM antioxidant ascorbic acid. Haloperidol (100 nM, 10 μM), quinpirole (10 μM), raclopride (10 μM), PD 128907 (100 nM), L-745870 (1 μM), tetrodotoxin (TTX, 1 μM), tetraethylammonium (TEA, 10 mM), and CGP 55845 (1 μM) were bath applied. All reagents were obtained from Sigma and freshly prepared from stock solutions.
Histological Procedures and 3D Computer Reconstruction. After recording, slices were fixed, stained, and 3D-computer reconstructed following the published procedures (19).
Statistical Analysis. Paired Student's t tests were performed for comparisons of EPSP amplitudes and stimulating strength under different pharmacological conditions.
Results
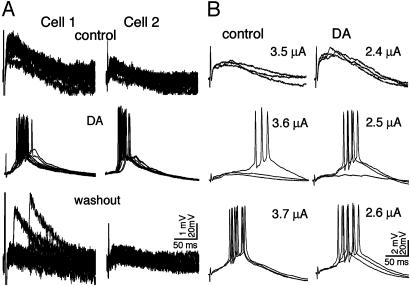
DA-Promoted Bursting. The effect of DA modulation was recorded from 248 layer V PCs under NMDA and GABAA blockade, 53 of which exhibited burst firing in response to extracellular stimulation in layer III. Bursts commonly occurred within 2-10 min of applying DA and gradually disappeared after washout of DA (Fig. 1A). As shown in Fig. 1B, DA also lowered the threshold for bursting by 1.5 μA on average (n = 25, SE = 0.5 μA; range, 0.4-11.0 μA; P < 0.005). In some experiments, we were able to record two PCs simultaneously (n = 27). The two neurons invariably exhibited remarkably similar temporal and spatial profiles of burst firing (Fig. 1 A), suggesting that these responses were components of synchronized activity in a neuronal circuit. It is worth mentioning here that DA-promoted bursting was also induced in the absence of NMDA blockade (n = 4), which exhibited different properties in terms of its reaction to haloperidol (see below).
Fig. 1.
DA-promoted bursting in layer V PCs. (A) Bursting responses promoted by DA (25 μM) with bath application of AP-5 (50 μM) and bicuculline (5 μM) to block NMDA and GABAA receptors, respectively. (Top) Before applying DA, 12 superimposed single non-NMDA EPSPs (0.1 Hz) simultaneously recorded from two layer V PCs. (Middle) Burst firing in all sweeps after applying DA for 3 min. (Bottom) Bursting disappeared after washout of DA for 15 min. (B) Bursting threshold reduced by DA. In control (Left), bursting was elicited at a stimulation strength of 3.6 μA. After applying DA (Right), bursting responses were triggered by stimulation at 2.5 μA. Additionally, the amplitudes of EPSPs evoked at a stimulating strength of 2.4 μA is higher than those evoked at a stimulating strength of 3.5 μA in control. Scale bars refer to EPSPs (small vertical calibrations) and APs (large vertical calibrations).
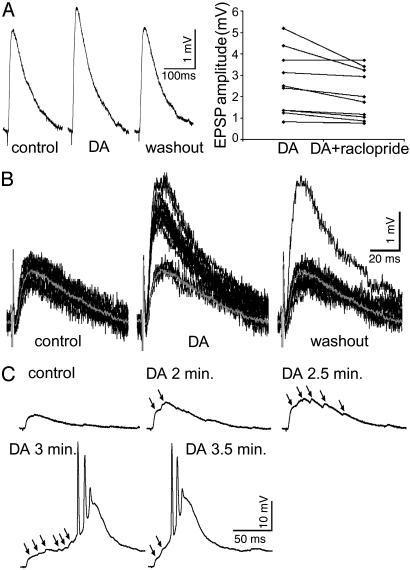
DA Enhancement of Non-NMDA EPSPs. Substantial heterogeneity of synaptic responses was observed among layer V PCs in their susceptibility and sensitivity to the DA application. Among the 248 recorded PCs, DA enhanced extracellularly evoked non-NMDA EPSPs in 48%, depressed them in 34%; and 18% of them were unchanged (defined by a change <10%). In the neurons in which DA had an enhancing effect, the amplitudes of the non-NMDA EPSPs were increased by 69% (SE, 15%; range, 12-673%; P < 0.001), and this enhancement was readily reversed by a D2 antagonist (raclopride, 10 μM; P < 0.03, n = 10) (Fig. 2A). The large increases in EPSP amplitude below bursting threshold further indicated that DA enabled the recruitment of excitatory connections. This possibility is exemplified by the neuron shown in Fig. 2B, which responded to 12 single pulses (0.1 Hz) with uniform EPSP amplitudes before DA application. After DA, the amplitudes of most responses doubled (some cases not shown increased up to 6-fold) to form a new group of uniform EPSPs, which recovered within 10 min after washout of DA. Furthermore, EPSPs evoked by a stimulating pulse generally exhibited a single peak reflective of a major monosynaptic EPSP in control. In responses to the same stimulating pulse, multiple peaks appeared after DA, and these responses gradually summated to reach bursting threshold (Fig. 2C). This progression from single peak to double or multiple peaks of EPSPs was observed in nearly half of the neurons (n = 59) in which DA enhanced non-NMDA EPSPs and in more than half of the cases in which DA promoted bursting (n = 31). We conclude from these several findings that DA-promoted bursts result from summation of DA enhanced non-NMDA EPSPs generated by recruited connections in addition to direct D2 enhancement of monosynaptic responses.
Fig. 2.
DA enhancement of monosynaptic non-NMDA EPSPs and recruitment of synaptic connections. (A) Monosynaptic non-NMDA EPSPs enhanced by DA and reversed by a D2 antagonist. (Left) EPSPs averaged over 12 sweeps (0.1 Hz) recorded before DA (left trace); after DA (10 min, middle trace); and after washout (25 min, right trace). The EPSP amplitude was increased by 19% when DA was applied, and recovered after washout of DA. (Right) Raclopride (10 μM) significantly reversed DA-enhanced EPSPs (n = 10, P < 0.03). (B) DA recruitment of synaptic connections at the monosynaptic level. Twelve single sweeps were superimposed respectively for control (Left), after DA (10 min, Middle), and after washout (10 min, Right). The gray traces in each panel are the averaged EPSP from 12 sweeps in control. By applying DA for 10 min, two groups of EPSP traces were formed: a few with amplitudes similar to that in control and most traces with twofold higher amplitudes. After washout of DA for 10 min, all amplitudes, except one, recovered. (C) DA recruitment of synaptic connections at the polysynaptic level. In control, the single peak EPSP presents major monosynaptic responses. After DA application, the peaks and amplitudes of the EPSPs gradually increased and facilitated (two peaks at 2 min, five peaks at 2.5 min, six peaks and bursting initiated at 3 min). At 3.5 min, the burst firing became strengthened as indicated by the shorter latency to bursting.
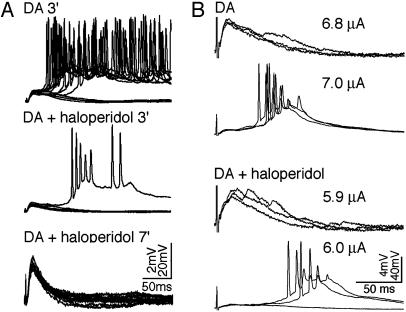
DA-Promoted Bursting Is Sensitive to Antipsychotic Drugs and D2 Antagonism. To examine the possibility that PC activation by DA observed under NMDA and GABAA blockade may share certain features of the pathophysiology of schizophrenia, we tested the ability of the typical antipsychotic drug haloperidol (10 μΜ, n = 13) to negate DA's effects. Haloperidol was highly effective in reversing the burst firing observed in PCs. Bath application of the drug dramatically attenuated DA-promoted bursting within 3-8 min and completely blocked it after 7-15 min (an example in Fig. 3A). Interestingly, in the absence of NMDA blockade, haloperidol exhibited an opposite effect by lowering burst threshold (n = 2 of 2, Fig. 3B).
Fig. 3.
Haloperidol presents opposite effects to DA-promoted bursts depending on the involvement of NMDA receptors. (A) Haloperidol attenuated DA-promoted bursts in the existence of NMDA receptor blockade. Bursting responses (most of 12 sweeps, 0.1 Hz) elicited after adding DA for 3 min (Top) were virtually eliminated (one bursting sweep; Left) after applying haloperidol (10 μM) for 3 min (Middle) and totally eliminated within 7 min (Bottom). (B) Haloperidol lowered DA bursting threshold in the absence of NMDA receptor blockade. By applying DA, DA-promoted bursts were initiated in the recorded cell with a stimulating strength of 7.0 μA (upper two rows). After applying haloperidol (100 nM), a 6.0 μA stimulus was enough to trigger the bursting (bottom two rows).
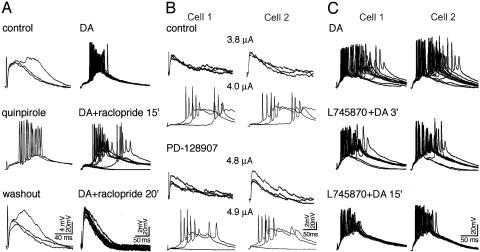
Although haloperidol has high affinity for D2 receptor sites (1, 2), it also has some affinities, especially at high concentrations, for other DA receptors such as D1 receptors (20). To clarify which DA receptors were involved in the induction of DA-promoted bursting, we chose highly selective agonists and antagonists of the D2 family receptors. The D2 agonist, quinpirole (10 μM), reproduced DA-promoted burst firing within 3-8 min of application (n = 5 of 5) (Fig. 4A Left), and the D2 antagonist, raclopride (10 μΜ), completely blocked DA-promoted bursting within 15-20 min (n = 4 of 4) (Fig. 4A Right). Raclopride was also effective in reversing DA enhancement of non-NMDA EPSPs (see Fig. 2 A), further implicating D2 receptor action as the essential mechanism of DA enhancement of non-NMDA EPSPs and DA-promoted bursts.
Fig. 4.
Distinctive effects of D2 family receptors on DA-promoted bursting. (A) D2 receptor agonist reproduced and D2 antagonist blocked DA bursts. (Left) Bursting induced by applying DA (data not shown) was then eliminated by washout of DA (Top). Quinpirole (10 μM) reproduced bursting within 3 min (Middle); the bursts disappeared after washout of quinpirole for 5 min (Bottom). (Right) Bursting responses induced by DA (25 μM, Top). Raclopride (10 μM) attenuated the bursts within 15 min, presenting no bursts in most sweeps (Middle), and completely blocked the bursts by 20 min (Bottom). (B) D3 receptor agonism increased the threshold for DA-promoted bursting. In control, bursting responses were initiated in both cells with a stimulating strength of 4.0 μA (upper two rows). After applying PD-128907 (100 nM), a 4.9-μA stimulus was required to trigger the bursting (bottom two rows). (C) D4 receptor antagonism could not block DA-promoted bursts. DA promoted bursts in all 12 sweeps (Top). Note that in some sweeps the bursts occurred after long latencies. Despite the application of L745870 (1μM) the bursting responses gradually became stronger indicated by gradually shorter latencies (Middle and Bottom). Note the similarity in pattern and time scale of bursting activity exhibited by the simultaneously recorded pairs of cells in B and C.
According to several studies, D3 agonists rather than D3 antagonists are antipsychotic (21, 22). Therefore, a selective D3 agonist, PD-128907 (100 nM), was tested in five cases in which DA-promoted bursts had recovered by washout. Bursting responses could not be reproduced in any of the five experiments. However, the bursting threshold was slightly increased in all five cases (mean ± SE, 0.6 ± 0.1 μA; range, 0.4-1.0 μA; P < 0.01), suggesting that a D3 agonist has a modest capability of attenuating bursting responses (Fig. 4B).
Although D4 receptors have been found to be abnormally increased in the brains of schizophrenia patients (23), D4 antagonists have rarely been reported to have antipsychotic efficacy. A selective D4 antagonist, L745870 (1 μM), was applied in five experiments in which DA-promoted bursts were recorded. In no case did we observe attenuation of bursting by L745870 applied for 15-20 min. In fact, bursting responses become more likely after applying L745870 as indicated by progressively shorter latencies to bursting in its presence (Fig. 4C).
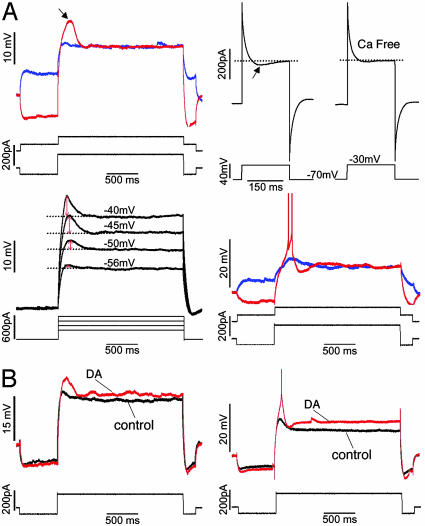
Layer V Intrinsically Bursting PCs Provide Cellular Basis for the DA-Promoted Bursts. Thirty-three of 56 PCs that exhibited DA-promoted bursts were stained well enough for the identification of their anatomical structure. All were distinguished by their thick-tufted apical dendrites that reached layer I, and by axons that were mainly distributed in layer V and VI. In addition, low-threshold spikes (LTS) formed by low-threshold, or T-type calcium currents (LTCC), were observed in all of these cells except for one. These cells likely correspond to the same layer V intrinsically bursting PCs described in a previous report (24). In accord with the literature, LTS was induced by a depolarizing pulse at hyperpolarized membrane potentials (-70 mV or more negative), but not at depolarized membrane potentials, and disappeared in calcium free bath solution (Fig. 5A Top). Applying TTX and TEA in the bath to block fast sodium spikes and potassium currents revealed that LTS became strengthened as the response potential became more and more depolarized (generally in a range from -60 mV to -40 mV). Intrinsic burst firing was more readily observed when LTS was triggered (Fig. 5A Middle). In addition, in line with previous studies (25-27), we show here that DA increases depolarizing currents, leading to enhancement of LTS and hence increased sensitivity to inputs that would be otherwise ineffective (Fig. 5B). Such mechanisms could therefore provide the essential cellular conditions for DA-promoted burst initiation.
Fig. 5.
Layer V bursting PCs presented LTS and their excitability was increased by DA. (A) LTS recorded from layer V bursting PCs. (Top Left) A LTS was clearly visible (arrow) in response to a positive pulse given at hyperpolarized membrane potential (red trace), but not at depolarized membrane potential (blue trace). (Top Right) Voltage clamp recording carried out in TTX and TEA bath revealed an inward current (arrow) by depolarizing from -70 mV to -30 mV (Left). This inward current tended to disappear in Ca2+ free bath (Right). (Middle Left) Using TTX and TEA bath blocking voltage gated Na+ and K+ channels, LTSs were visible at the different potentials by giving stepped positive currents. LTS amplitudes (red arrows) were gradually increased when the potentials were gradually depolarized (from -56 mV to -40 mV). (Middle Right) A two-AP burst was triggered when LTS was induced (red trace) but not when LTS was not induced (blue trace). (B) DA increased the excitability of layer V bursting PCs, leading enhancement of LTS. (Bottom Left) The response potential in DA was more depolarized (red trace) compared to control (black trace). Correspondingly, the LTS amplitude of the DA sweep was obviously higher than that of control. (Bottom Right) Before giving DA (black trace), no AP was triggered by a depolarizing pulse. After applying DA (red trace), the response potential became more depolarized and an AP was triggered by the same stimulation pulse as in control. All of the responses were recorded at membrane potential of -70 mV, and all of the stimulating sweeps were presented below the response traces. These sweeps were collected from four PCs. For clearer presentation, APs were partly truncated.
Discussion
The present experiments have revealed that DA, acting mainly at D2 receptors, can promote bursting in layer V PCs in response to minimal extracellular stimulation in layer III under NMDA and GABAA blockade. This DA-promoted bursting was reproduced by a D2 selective agonist, and reversed by the typical antipsychotic drug haloperidol as well as by a D2 receptor antagonist, and a D3 agonist but not a D4 antagonist. These findings and their implications for the pathophysiology of schizophrenia are discussed below.
DA Enhancement at Non-NMDA Receptors. D2 receptor excitatory mediation of glutamatergic receptors has been implicated in previous in vivo studies (3, 4). Fewer studies of D2 receptor action have been carried out in cortical slices, and those studies have found that this receptor subtype depressed excitatory transmission (5, 6, 8). The present study shows that D2 receptor stimulation can induce enhancement of non-NMDA EPSPs and promote bursts in prefrontal cortical PCs in vitro. The depressive effects observed in vitro possibly reflected D2 heteroreceptor activation at presynaptic sites, which would be expected to reduce rather than enhance excitatory synaptic transmission (28). Depressive effects of DA on non-NMDA activity may also predominate in vitro because of the higher density (29) and affinity (30) of D1 receptors in many cortical and subcortical areas (10, 18). More importantly, different components of glutamatergic synaptic responses evoked by extracellular stimulation are often differentially modulated by D1 and D2 receptors (5, 7-10). The recorded change is actually a combination of different changes from these components, possibly masking specific D1 or D2 actions on a glutamatergic receptor type. Previous in vitro experiments designed to isolate non-NMDA currents might not have completely eliminated NMDA currents by using methods such as clamping membrane potentials close to resting levels (31, 32). In the present study, non-NMDA receptor activity was well isolated by applying NMDA and GABAA antagonists, and the stimulation strength was controlled at minimal level to evoke a stable EPSP. The components of EPSPs were therefore only of non-NMDA origin, and were further minimized by stimulating a few or even a single synaptic connection, maximally limiting the synaptic response components that were differentially modulated by DA. In this way, it became possible to reveal DA enhancement at non-NMDA EPSPs mainly via D2 receptors in a set of layer V PCs. Commonly, the enhancement of neuronal activity involves direct action on synapses, which can be facilitated by increased cell excitability (33). In the present study, the DA enhancement of non-NMDA EPSPs likely involved direct D2 effects on synapses of the involved connections because D2 action itself inhibits the excitability of layer V PCs in PFC (34). Furthermore, this enhancement of non-NMDA receptor action, in contrast to DA depression via modulation of D2 presynaptic sites, may implicate postsynaptic actions.
DA-Promoted Bursting Reflects Recruitment of Excitatory Inputs. In addition to the direct enhancement of non-NMDA EPSPs, DA also led to recruitment of synaptic connections. Due to enhanced depolarizing currents induced by DA and indirectly enhanced LTS (Fig. 5B), a stimulating pulse in layer III, which normally triggered subthreshold responses, might become sufficient to cause a PC to reach firing and/or bursting threshold. The increased PC excitability and direct enhancement of non-NMDA EPSPs by DA (Fig. 2 A) made recruitment of more synaptic connections possible. The uniformity (Fig. 2B) and striking 2- to 6-fold increase in amplitude in the presence of DA is consistent with recruitment of monosynaptic connections as is the EPSP shape change from single peak before DA to multiple peaks after DA (Fig. 2C), consistent with recruitment of polysynaptic connections. Once activated, PCs undoubtedly could excite other neurons and set in motion of a cascade of recurrent excitation within the PC-PC connectivity, creating a high degree of synchronization in the local neuronal circuit. Several lines of evidence support such synchronized neuronal activity. First, burst firing did not have consistent onset latency, suggesting that the burst was mediated by polysynaptic activation. Second, simultaneously recorded pairs of neurons exhibited responses with very similar spatial and temporal patterns, indicating that the activities of these pairs were synchronized (examples as in Figs. 1 A and 4 B and C). Therefore, DA was able to promote subthreshold responses to synchronized network action.
Members of the D2 Family Are Distinct in Their Effects on DA-Promoted Bursting. Although D2, D3, and D4 receptors share similar structural, pharmacological, and biochemical properties (35), apparently they differ in their physiological actions and, as shown here, in their capacity for promoting burst firing (Fig. 4). Only a selective D2 receptor agonist, quinpirole, was able to reproduce the burst firing promoted by DA, and a D2-selective antagonist, raclopride, blocked DA-promoted bursts in all cases examined. In sharp contrast, a D3 agonist increased the threshold for DA-promoted bursts, and a D4 antagonist even enhanced DA-promoted bursts.
In line with previous studies, the present findings on induction of DA-promoted bursts mainly via D2 action may be in accord with a postsynaptic mechanism of neuronal activation (3). This mechanism is further supported by the recent report that haloperidol is ineffective in mice lacking the D2L isoform that is located on postsynaptic membranes, but is still effective in mice lacking the D2S isoform predominantly located presynaptic membranes (36). In contrast, both D3 (37) and D4 (38) receptors have been more often associated with depression of excitatory transmission. Thus, it is reasonable to conclude that the nature of DA modulation of neuronal responses to excitatory input is largely determined by which D2 family member is predominantly involved pre- and/or postsynaptically in the activated connections. Similar considerations may apply to D1 family members and receptors of other neuronal modulators like 5-hydroxytryptamine (5-HT). Finally, it is conceivable that either enhancement or suppression of DA will depend on combinatorial effects involving all these receptor actions.
Intrinsically Bursting PCs with Low-Threshold Calcium Currents (LTCC) and DA-Promoted Bursting. It is of considerable interest that in the present study the PCs that responded to DA by bursting are those that have previously been characterized by the presence of large delayed inhibitory postsynaptic potentials (IPSPs) (presumed GABAB IPSPs) and LTSs (24). It may be the concatenation of these two features that render the layer V bursting PCs particularly sensitive to DA and DA-promoted bursts. DA is known to increase persistent Na+ currents (25, 27), thereby further depolarizing membrane potentials and exposing later IPSPs (24). The hyperpolarization produced by the later IPSPs could enable the deinactivation of LTCC and hence initiation of LTS that could subsequently be enhanced by the DA-mediated increase in persistent Na+ current. Finally, the PCs fired more easily and the intrinsic bursts were easily initiated from these neurons. Indeed, DA-promoted bursting could also be completely blocked by GABAB blockade (CGP 55845, 1 μM; n = 3 of 3, data not shown), supporting such a hypothesized processing. Considering that intrinsically bursting PCs with LTS are found only in layers V and VI in PFC (24), that synchronized activity in cortex is initiated from layer V (39), and that D2 receptor density is highest in layer V compared to other layers in the PFC (29, 40), this PC type possibly serves as the cellular basis for the synchronized bursting activity under normal conditions.
Summarizing the above evidence, the induction of bursting by DA is related to at least three possible mechanisms: DA enhancement of monosynaptic non-NMDA EPSPs mainly via D2 action, DA recruitment of synaptic connections, and DA mediated increased excitability of layer V intrinsically bursting PCs. GABAA blockade in the study would also contribute to DA-promoted bursting by reducing inhibitory tone in the neuronal network (41). In view of the fact that DA-promoted bursting was recorded only in a small subset of PCs, DA effects on excitatory transmission in PFC may be pathway specific, similar to previous studies (42). In addition, the fact that burst firing was promoted by DA in the absence of NMDA receptor blockade indicates that DA-promoted burst induction is independent of NMDA antagonism. However, the DA-promoted bursts involving NMDA action was enhanced rather than blocked by haloperidol, suggesting another kind of DA-promoted bursting with different biophysiological significance from the DA-promoted bursts involving only non-NMDA receptors. It would be interesting to explore the difference between these two kinds of DA-promoted bursts in future.
Significance of DA-Promoted Bursts. The bursting responses promoted by DA and D2 receptor agonists was reversed by haloperidol, a D2 antagonist and a D3 agonist, compounds having antipsychotic efficacy. In contrast, a D4 antagonist has not proven clinically effective and one tested here was not effective in blocking DA-promoted bursts. The remarkably similar profiles of response to pharmacological agents of DA-promoted bursts evidenced in the present in vitro study and clinical efficacy of antipsychotics for symptoms in schizophrenia may suggest some potential significance of DA-promoted bursts to this disease.
The first, and most important implication, is that psychotic symptoms (possibly positive symptoms) may result from the supersensitivity of a subset of cortical pyramidal neurons to excessive activation, including burst firing by stimulation of D2 receptors. Recent postmortem evidence of an up-regulation of NCS-1, a neuron calcium-sensing protein, is in accord with this hypothesis because NCS-1 interacts selectively with D2 receptors and serves to retain them at the plasma membrane (43). Thus, its up-regulated state in tissue from medicated patients may be an indication of a chronic state of D2 sensitization. The elevated D2 action also reduces inhibitory transmission by reducing release probability of GABAergic connections (44), further facilitating the supersensitivity of the particular subset of PCs.
The present findings also have the potential to explain why the affinity of typical antipsychotic drugs for the D2 receptor predicts their clinical efficacy. The data indicates that it is due to the ability of D2 blockade to normalize the response of abnormally sensitized PCs to extrinsic excitatory inputs and prevent them from being catapulted into a burst-firing mode. In contrast to D2 antagonists and not withstanding evidence of D4 receptor up-regulation in the schizophrenic brain (23, 45), D4 antagonists were not able to block the bursting and have proven ineffective and even worsen symptoms in some cases (46, 47). D4 receptors may play a role in the formation of NMDA hypofunction as suggested in a recent slice study (48), a possibility that is, however, beyond the scope of the present study because of the NMDA-blocking scheme in the experiments.
Finally, the present results could probably question the long-held view that hyperactivity at striatal D2 receptors is largely responsible for positive symptoms in schizophrenia. The direct study of prefrontal neurons clearly indicates that hyperactivity of D2 receptors may also exist in cortical areas, at least in the PFC, in addition to subcortical areas as revealed by numerous previous studies (49, 50, ‡). This hypothesis is supported by the fact that dopamine D2 mRNAs were abnormally elevated in frontal cortex of schizophrenia (51). Furthermore, a recent study of amphetamine sensitization in nonhuman primates suggested that the hyperactivity of D2 receptors in prefrontal cortex might be responsible for the nonmotoric positive symptoms of schizophrenia such as delusions and hallucinations. Amphetamine readily sensitized positive-like symptoms that were observed in normal animals but not in monkeys with prefrontal ablations, who instead showed only the hyperlocomotor responses to amphetamine that are associated with striatal stimulation (52).
Acknowledgments
We are grateful to Drs. D. McCormick, S. D. Antic, T. Koos, and W. J. Gao for comments on the manuscript and to Mariamma Pappy and Anita Begovic for technical assistance. This work was supported by National Institute of Mental Health Grant RO1 MH38546.
Abbreviations: DA, dopamine; NMDA, N-methyl-D-aspartate; GABA, γ-aminobutyric acid; PFC, prefrontal cortex; PC, pyramidal cell; EPSP, excitatory synaptic potential; TTX, tetrodotoxin; TEA, tetramethylammonium; LTS, low-threshold spikes.
Footnotes
Laruelle, M. (2003) in Glutamate and Disorders of Cognition and Motivation, eds. Moghaddam, B. & Wolf, M. E. (Yale Univ., New Haven, CT), p. 14 (abstr.).
References
- 1.Seeman, P. & Lee, T. (1975) Science 188, 1217-1219. [DOI] [PubMed] [Google Scholar]
- 2.Creese, I., Burt, D. R. & Snyder, S. H. (1976) Science 192, 481-483. [DOI] [PubMed] [Google Scholar]
- 3.Del Arco, A. & Mora, F. (2002) Brain Res. Bull. 57, 623-630. [DOI] [PubMed] [Google Scholar]
- 4.Wang, M., Vijayraghavan, S. & Goldman-Rakic, P. S. (2004) Science 303, 853-856. [DOI] [PubMed] [Google Scholar]
- 5.Levine, M. S., Li, Z., Cepeda, C., Cromwell, H. C. & Altemus, K. L. (1996) Synapse 24, 65-78. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda, C. & Levine, M. S. (1998) Dev. Neurosci. 20, 1-18. [DOI] [PubMed] [Google Scholar]
- 7.Zheng, P., Zhang, X. X., Bunney, B. S. & Shi, W. X. (1999) Neuroscience 91, 527-535. [DOI] [PubMed] [Google Scholar]
- 8.Urban, N. N., Gonzalez-Burgos, G., Henze, D. A., Lewis, D. A. & Barrionuevo, G. (2002) J. Physiol. 539, 707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cepeda, C., Radisavljevic, Z., Peacock, W., Levine, M. S. & Buchwald, N. A. (1992) Synapse 11, 330-341. [DOI] [PubMed] [Google Scholar]
- 10.Seamans, J. K., Durstewitz, D., Christie, B. R., Stevens, C. F. & Sejnowski, T. J. (2001) Proc. Natl. Acad. Sci. USA 98, 301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abi-Saab, W. M., D'Souza, D. C., Moghaddam, B. & Krystal, J. H. (1998) Pharmacopsychiatry 31, 104-109. [DOI] [PubMed] [Google Scholar]
- 12.Olney, J. W., Newcomer, J. W. & Farber, N. B. (1999) J. Psychiatr. Res. 33, 523-533. [DOI] [PubMed] [Google Scholar]
- 13.Jentsch, J. D. & Roth, R. H. (1999) Neuropsychopharmacology 20, 201-225. [DOI] [PubMed] [Google Scholar]
- 14.Tsai, G. & Coyle, J. T. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 165-179. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds, G. P. & Beasley, C. L. (2001) J. Chem. Neuroanat. 22, 95-100. [DOI] [PubMed] [Google Scholar]
- 16.Beasley, C. L., Zhang, Z. J., Patten, I. & Reynolds, G. P. (2002) Biol. Psychiatry 52, 708-715. [DOI] [PubMed] [Google Scholar]
- 17.Goldman-Rakic, P. S. & Selemon, L. D. (1997) Schizophr. Bull. 23, 437-458. [DOI] [PubMed] [Google Scholar]
- 18.Gao, W. J., Krimer, L. S. & Goldman-Rakic, P. S. (2001) Proc. Natl. Acad. Sci. USA 98, 295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, Y., Gupta, A., Toledo-Rodriguez, M., Wu, C. Z. & Markram, H. (2002) Cereb. Cortex 12, 395-410. [DOI] [PubMed] [Google Scholar]
- 20.Meltzer, H. Y., Matsubara, S. & Lee, J. C. (1989) J. Pharmacol. Exp. Ther. 251, 238-246. [PubMed] [Google Scholar]
- 21.Witkin, J., Gasior, M., Acri, J., Beekman, M., Thurkauf, A., Yuan, J., DeBoer, P., Wikstrom, H. & Dijkstra, D. (1998) Eur. J. Pharmacol. 347, R1-R3. [DOI] [PubMed] [Google Scholar]
- 22.Fink-Jensen, A. (2000) Dan. Med. Bull. 47, 151-167. [PubMed] [Google Scholar]
- 23.Lahti, R. A., Roberts, R. C., Cochrane, E. V., Primus, R. J., Gallager, D. W., Conley, R. R. & Tamminga, C. A. (1998) Mol. Psychiatry 3, 528-533. [DOI] [PubMed] [Google Scholar]
- 24.de la Pena, E. & Geijo-Barrientos, E. (1996) J. Neurosci. 16, 5301-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, C. R. & Seamans, J. K. (1996) J. Neurosci. 16, 1922-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi, W. X., Zheng, P., Liang, X. F. & Bunney, B. S. (1997) Synapse 26, 415-422. [DOI] [PubMed] [Google Scholar]
- 27.Gorelova, N. A. & Yang, C. R. (2000) J. Neurophysiol. 84, 75-87. [DOI] [PubMed] [Google Scholar]
- 28.Pothos, E. N., Przedborski, S., Davila, V., Schmitz, Y. & Sulzer, D. (1998) J. Neurosci. 18, 5575-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lidow, M. S., Goldman-Rakic, P. S., Gallager, D. W. & Rakic, P. (1991) Neuroscience 40, 657-671. [DOI] [PubMed] [Google Scholar]
- 30.Seeman, P. (1986) Drug Dev. Res. 9, 63-69. [Google Scholar]
- 31.Harvey, J. & Lacey, M. G. (1997) J. Neurosci. 17, 5271-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cepeda, C., Colwell, C. S., Itri, J. N., Chandler, S. H. & Levine, M. S. (1998) J. Neurophysiol. 79, 82-94. [DOI] [PubMed] [Google Scholar]
- 33.Koester, J. (1991) in Principles of Neural Science, eds. Kamdel, E. R., Schwartz, J. H. & Jessell, T. M. (Elsevier Science, New York), p. 95.
- 34.Gulledge, A. T. & Jaffe, D. B. (1998) J. Neurosci. 18, 9139-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neve, K. A. & Neve, R. L. (1996) The Dopamine Receptors (Humana, Totowa, NJ).
- 36.Usiello, A., Baik, J. H., Rouge-Pont, F., Picetti, R., Dierich, A., LeMeur, M., Piazza, P. V. & Borrelli, E. (2000) Nature 408, 199-203. [DOI] [PubMed] [Google Scholar]
- 37.Svensson, K., Carlsson, A., Huff, R. M., Kling-Petersen, T. & Waters, N. (1994) Eur. J. Pharmacol. 263, 235-243. [DOI] [PubMed] [Google Scholar]
- 38.Rubinstein, M., Cepeda, C., Hurst, R. S., Flores-Hernandez, J., Ariano, M. A., Falzone, T. L., Kozell, L. B., Meshul, C. K., Bunzow, J. R., Low, M. J., et al. (2001) J. Neurosci. 21, 3756-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Vives, M. V. & McCormick, D. A. (2000) Nat. Neurosci. 3, 1027-1034. [DOI] [PubMed] [Google Scholar]
- 40.Goldman-Rakic, P. S., Lidow, M. S. & Gallager, D. W. (1990) J. Neurosci. 10, 2125-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulledge, A. T. & Jaffe, D. B. (2001) J. Neurophysiol. 86, 586-595. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Islas, C. & Hablitz, J. J. (2003) J. Neurosci. 23, 867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koh, P. O., Undie, A. S., Kabbani, N., Levenson, R., Goldman-Rakic, P. S. & Lidow, M. S. (2003) Proc. Natl. Acad. Sci. USA 100, 313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seamans, J. K., Gorelova, N., Durstewitz, D. & Yang, C. R. (2001) J. Neurosci. 21, 3628-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeman, P., Guan, H. C. & Van Tol, H. H. (1993) Nature 365, 441-445. [DOI] [PubMed] [Google Scholar]
- 46.Bristow, L. J., Kramer, M. S., Kulagowski, J., Patel, S., Ragan, C. I. & Seabrook, G. R. (1997) Trends Pharmacol. Sci. 18, 186-188. [DOI] [PubMed] [Google Scholar]
- 47.Truffinet, P., Tamminga, C. A., Fabre, L. F., Meltzer, H. Y., Riviere, M. E. & Papillon-Downey, C. (1999) Am. J. Psychiatry 156, 419-425. [DOI] [PubMed] [Google Scholar]
- 48.Kotecha, S. A., Oak, J. N., Jackson, M. F., Perez, Y., Orser, B. A., Van Tol, H. H. & MacDonald, J. F. (2002) Neuron 35, 1111-1122. [DOI] [PubMed] [Google Scholar]
- 49.Carter, C. J. & Pycock, C. J. (1980) Brain Res. 192, 163-176. [DOI] [PubMed] [Google Scholar]
- 50.Deutch, A. Y. (1992) J. Neural Transm. Suppl. 36, 61-89. [DOI] [PubMed] [Google Scholar]
- 51.Tallerico, T., Novak, G., Liu, I. S., Ulpian, C. & Seeman, P. (2001) Brain Res. Mol. Brain Res. 87, 160-165. [DOI] [PubMed] [Google Scholar]
- 52.Castner, S. A. & Goldman-Rakic, P. S. (2003) Biol. Psychiat. 54, 105-110. [DOI] [PubMed] [Google Scholar]