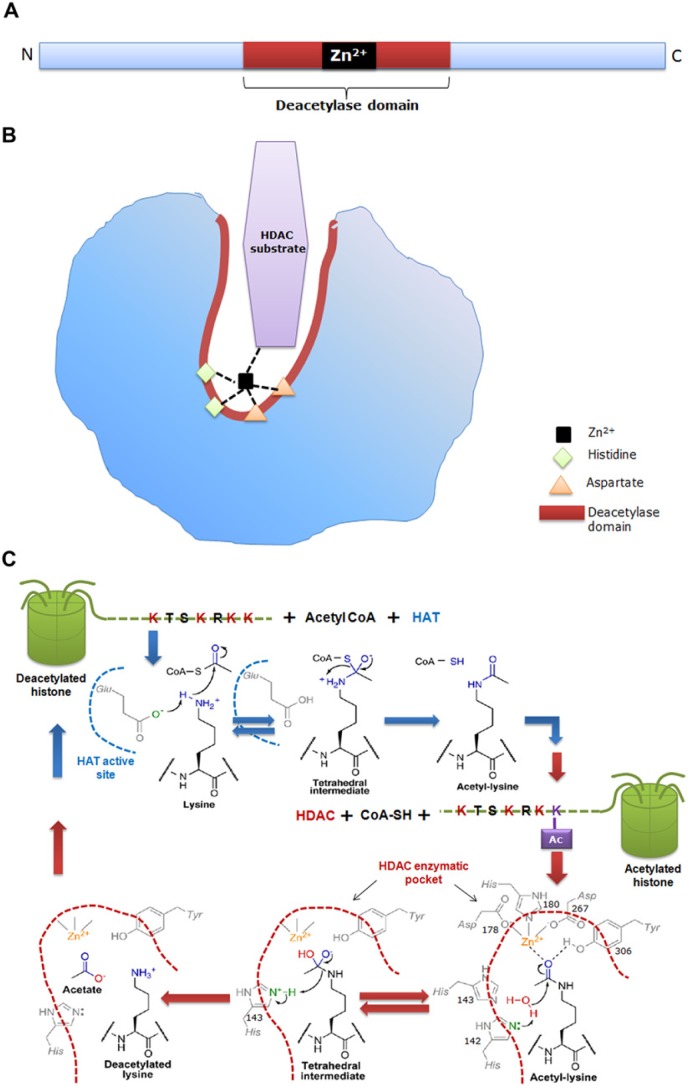
Figure 2.
(a) Schematic representation of the primary structure of a classical histone deacetylase (HDAC) enzyme, depicting the catalytic site as red/brown box and a Zn2+ site as a black box. (b) Schematic representation of the 3-dimensional structure of the HDAC showing a narrow, tubular catalytic pocket with a Zn2+ near its base. Zn2+, with the aid of histidine-aspartate charge relay system, imparts catalytic activity to the HDAC enzyme. Black dotted lines represent charge relay between histidine, aspartate residues and HDAC substrate. (c) Detailed mechanism of the histone acetylase (HAT) and HDAC enzymatic reaction. During acetylation, the active site glutamate (Glu) of HAT acts as general base to activate the e-amine group of lysine, which helps in the nucleophilic attack of the carbonyl carbon within acetyl-CoA, thus producing a tetrahedral intermediate. Subsequently, the tetrahedral intermediate collapses to form acetylated lysine and CoA. The active site of HDAC harbors His, Asp and Tyr residues. In deacetylation, both His-142 and His-143 can act as general bases by donating a pair of electrons to the water molecule to activate it for nucleophilic attack of the acetyl carbonyl carbon. An attack by water results in a tetrahedral intermediate, which is stabilized by Zn2+ and Tyr-306. Subsequently, the tetrahedral intermediate collapses to form acetate and lysine products, with His-143 acting as a general acid to protonate the e-amine leaving group of lysine. Blue dotted line depicts the active site of HAT and brown dotted line represents the tubular pocket of active site of HDAC. (Chemical structures adopted from Smith & Denu, 2009).