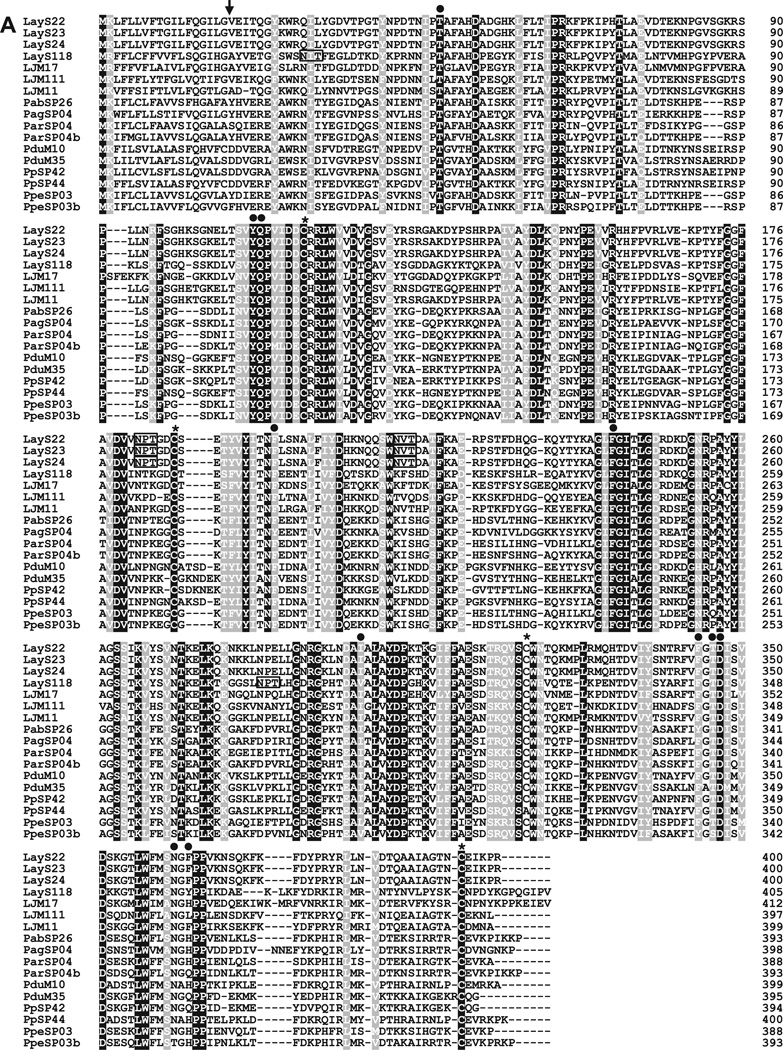
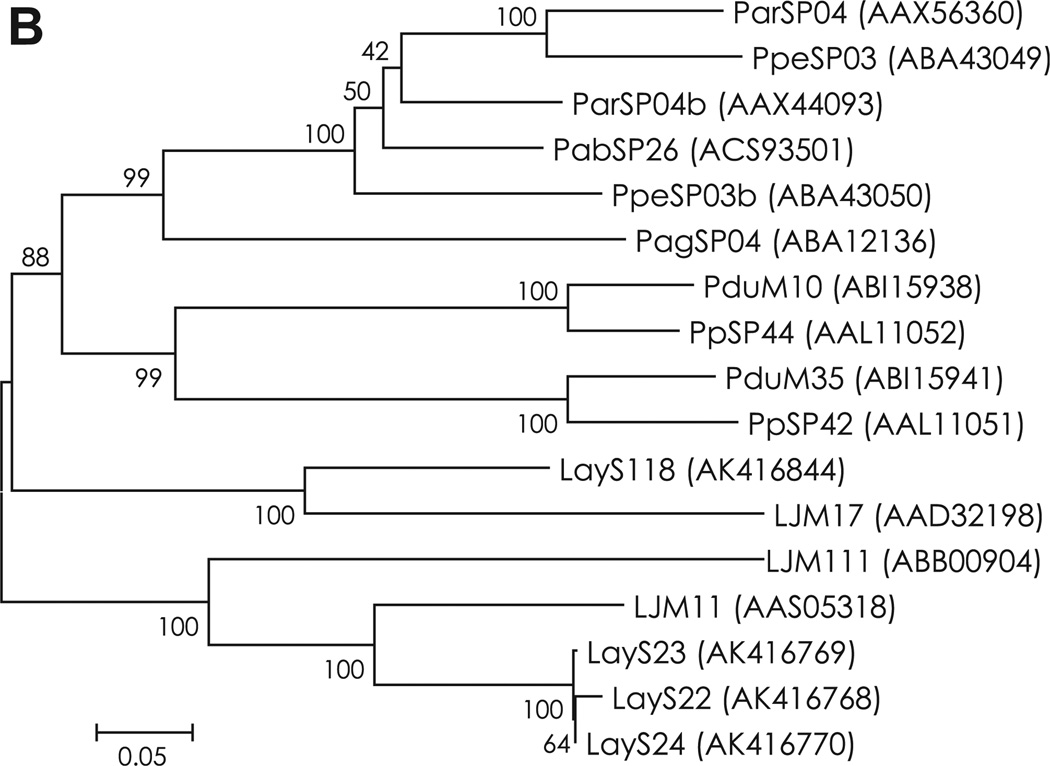
Fig. 2.
(A) Sequence alignment of yellow-related proteins from Lu. ayacuchensis (LayS22, LayS23, LayS24, and LayS118) together with those from P. arabicus (Pab), P. argentipes (Pag), P. ariasi (Par), P. duboscqi (Pdu), P. papatasi (Pp), P. perniciosus (Ppe), and Lu. longipalpis (LJM). Black-shaded amino acids represent identical amino acids and gray-shaded amino acids represent conserved amino acids. Dashes indicate gaps introduced for maximal alignment. Asterisks at the top of the amino acids denote conserved cysteine residues, and an arrowhead indicates the predicted signal peptide cleavage site. Closed circles show conserved amino acids contained in the ligand binding pocket of yellow-related protein family. Potential N-glycosylation sites of Lu. ayacuchensis yellow-related proteins are boxed. (B) Phylogenetic tree analysis of amino acid sequences of yellow-related proteins from sand flies. Accession numbers are in parentheses and node values indicate branch support.