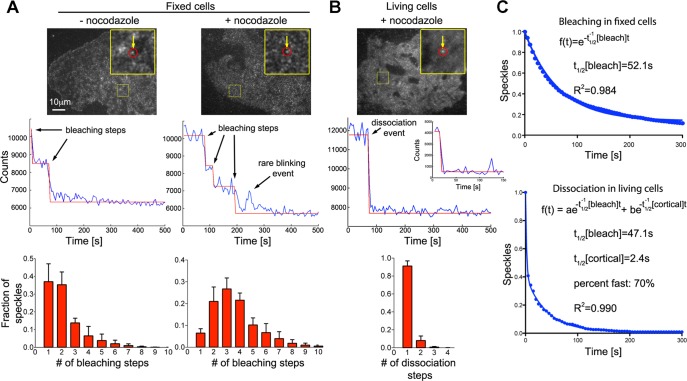
FIGURE 1:
Rapid turnover of dynein complexes at the cell cortex. (A) Bleaching of individual EGFP-labeled dynein heavy-chain (Dync1h1) speckles in fixed COS7 cells (top) shows stepwise decrease in fluorescence intensity (middle). Fitting of a step function to the bleaching kinetics allows estimation of the number of EGFP molecules per speckle, which is displayed as histograms (bottom). After overnight depolymerization of microtubules with 10 μM nocodazole (n = 4896 speckles in four cells), the distribution of bleaching steps per speckle is shifted toward larger numbers compared with control fixed cells (n = 1982 speckles in three cells). (B) Rapid dissociation of EGFP-labeled dynein heavy-chain (Dync1h1) speckles from the cell cortex in living COS7 cells (top). Middle, to best illustrate the steplike dissociation, an unusually stable speckle that dissociates from the cortex within a single video frame after a prolonged delay is shown. The corresponding inset shows the more frequent, rapid dissociation within the first acquired video frames. The distribution of dissociation steps shows that speckles usually dissociate in a single step (bottom left; n = 1599 speckles in four cells). (C) Number of remaining EGFP-Dync1h1 molecules plotted against time. In fixed cells, the bleaching kinetics of initially detected individual EGFP molecules fits well to a single-exponential decay function (the average value was t1/2[bleach] = 46.2 ± 2.0 s, n = 4896 speckles in four cells). In living cells, the kinetics of EGFP-Dync1h1 dissociation does not fit a single-exponential decay (R = 0.94 ± 0.02). Assuming similar bleaching kinetics in fixed and living cells, a fast component, which is due to dynamic interaction of dynein speckles with the cortex, is detected using a double-exponential fit (t1/2[cortical] = 2.6 ± 0.9 s, fraction fast: 59 ± 13%, n = 1599 speckles in four cells; the t1/2 of the slow component was set to the average t1/2[bleach] obtained in fixed cells from the same experimental day). The overall density of speckles was 0.28 ± 0.05 (live cells) or 0.46 ± 0.13 (fixed cells) speckles/μm2 (n = 6 or 4 cells).