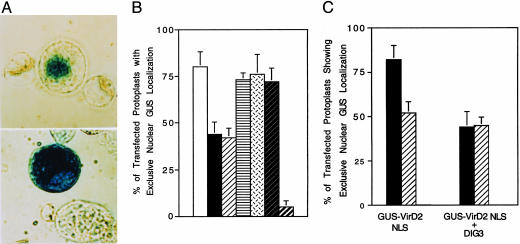
Fig. 5.
(A) Expression of the DIG3 cDNA in tobacco BY-2 protoplasts inhibits nuclear import of a GUS VirD2 NLS fusion protein. Fourteen hours after electroporation, protoplasts were stained with 5-bromo-4-chloro-3-indolyl β-d-glucuronide (X-gluc) and visualized by using a phase-contrast microscope. (Upper) Electroporation of a GUS-VirD2 NLS fusion gene. (Lower) Coelectroporation of DIG3 and a GUS-VirD2 NLS fusion gene. (B) Percentage of transfected protoplasts with GUS activity localized exclusively in the nucleus. Approximately 100 X-glucstained protoplasts were scored for each sample, and each experiment was performed three times in molar ratios of 10:1 and 20:1. Coelectroporation controls, vector, and GUSVirD2 NLS or DIG3 and GUS-NIa were performed at a ratio of 20:1. □, GUS-VirD2 NLS; ▪, DIG3 + GUS-VirD2 NLS (10:1);  , DIG3 + GUS-VirD2 NLS (20:1);
, DIG3 + GUS-VirD2 NLS (20:1);  , vector + GUS-VirD2 NLS (20:1);
, vector + GUS-VirD2 NLS (20:1);  , GUS-NIa;
, GUS-NIa;  , DIG3 + GUS-NIa;
, DIG3 + GUS-NIa;  , GUS. (C) A serine residue near the VirD2 C-terminal NLS is involved in nuclear import. Tobacco BY-2 protoplasts were electroporated with either the GUS-VirD2 NLS constructions alone or with the DIG3 gene. X-gluc-stained protoplasts were scored for the percentage of protoplasts that showed exclusive nuclear GUS localization. At least 300 transfected protoplasts were examined for each sample. ▪, GUS-VirD2 NLS;
, GUS. (C) A serine residue near the VirD2 C-terminal NLS is involved in nuclear import. Tobacco BY-2 protoplasts were electroporated with either the GUS-VirD2 NLS constructions alone or with the DIG3 gene. X-gluc-stained protoplasts were scored for the percentage of protoplasts that showed exclusive nuclear GUS localization. At least 300 transfected protoplasts were examined for each sample. ▪, GUS-VirD2 NLS;  , GUS-VirD2 NLS (Ser-394→Ala).
, GUS-VirD2 NLS (Ser-394→Ala).