Abstract
Cotton plants are subjected to the attack of several insect pests. In Brazil, the cotton boll weevil, Anthonomus grandis, is the most important cotton pest. The use of insecticidal proteins and gene silencing by interference RNA (RNAi) as techniques for insect control are promising strategies, which has been applied in the last few years. For this insect, there are not much available molecular information on databases. Using 454-pyrosequencing methodology, the transcriptome of all developmental stages of the insect pest, A. grandis, was analyzed. The A. grandis transcriptome analysis resulted in more than 500.000 reads and a data set of high quality 20,841 contigs. After sequence assembly and annotation, around 10,600 contigs had at least one BLAST hit against NCBI non-redundant protein database and 65.7% was similar to Tribolium castaneum sequences. A comparison of A. grandis, Drosophila melanogaster and Bombyx mori protein families’ data showed higher similarity to dipteran than to lepidopteran sequences. Several contigs of genes encoding proteins involved in RNAi mechanism were found. PAZ Domains sequences extracted from the transcriptome showed high similarity and conservation for the most important functional and structural motifs when compared to PAZ Domains from 5 species. Two SID-like contigs were phylogenetically analyzed and grouped with T. castaneum SID-like proteins. No RdRP gene was found. A contig matching chitin synthase 1 was mined from the transcriptome. dsRNA microinjection of a chitin synthase gene to A. grandis female adults resulted in normal oviposition of unviable eggs and malformed alive larvae that were unable to develop in artificial diet. This is the first study that characterizes the transcriptome of the coleopteran, A. grandis. A new and representative transcriptome database for this insect pest is now available. All data support the state of the art of RNAi mechanism in insects.
Introduction
Insects comprise more than one million of the described species. Despite the great diversity of species and the importance of insects, mainly as disease vectors and agricultural pests, attainable molecular biology resources for insect study still need to be increased in order to understand their physiology and biochemistry and to find new targets for biotechnological tools. At least one-third of insect species are beetles, making coleopterans the most diverse order of living organisms. Nevertheless, few data are available for coleopteran molecular resources in the current available databases. The NCBI nucleotide database (http://www.ncbi.nlm.nih.gov/nuccore/), for example, has currently around 340,000 of total sequences for beetles. Only eight coleopteran species have more than 5,000 sequences deposited: Pogonus chalceus (65779), Dendroctonus ponderosae (41429), Rhynchophorus ferrugineus (27014), Dendroctonus frontalis (20987), T. castaneum (16808), Ips typographus (14810), Agrilus planipennis (12018), A. grandis (5705). Moreover, no more than 50 insect complete genomes are available for blast tool nucleotide search. From these, just one, T. castaneum, is coleopteran. Recently, a consortium called the i5k Initiative, also known as the 5,000 Insect Genome Project, was recently launched and aims to sequence the genomes of all insect species known to be important to worldwide agriculture, food safety, medicine, and energy production [1].
In the last years, the study of insect transcriptomes has been employed to evaluate gene expression profile for biotechnological use. After the introduction of Next Generation Sequencing techniques largely used for genome sequencing, transcriptome analysis became fast and efficient, and it is currently possible to detect and identify microRNAs, 3’ and 5’ untranslated regions and even complete mRNAs [2,3]. Particularly, the next generation sequencing strategy of pyrosequencing using 454 [4,5] has been used to study insect vectors [6–9] and to assess insect genes that code for hemolymph and midgut proteins [10–12], metabolic pathway enzymes [13–15] and metagenomics [16]. Moreover, some transcriptome studies were made in order to provide ESTs datasets of model and non-model insects [9,17–19] and have become an effective way of assessing gene expression levels.
The cotton boll weevil, A. grandis, has been the most important cotton insect-pest in North America. Due to the Boll Weevil Eradication Program, sponsored by the USDA, an integrated pest management strategy has been successful in controlling boll weevil populations [20]. In South America, nevertheless, the insect populations are still causing great damage to the cotton crops, destroying cotton plant floral buds and bolls. Due to their high reproductive rate in tropical areas and to the endophytic behavior of earlier developmental stages, infestation levels increase fast and unless control measures are adopted, damages can lead up to total loss of production [21]. The ineffectiveness and harmful aspects in using chemical control to arrest the infestation leads to the search for more efficient control strategies, of which the most promising are in the biotechnological area.
The use of genetically modified (GM) crops to control insect pests is now widely used. Several proteins have been introduced in plants in order to control insects, mainly the Bacillus thuringiensis (Bt) toxins [22]. None is reported to control cotton boll weevil. The use of double-stranded RNA (dsRNA) to silence gene expression is currently a highly explored approach to generate insect-resistant genetically GM crops [23–25]. Moreover, RNAi is tool widely used in reverse genetics studies. Recent results showed the viability of the use of dsRNA-producing plants as an insect-pest control approach. Two groups reported GM plants that express dsRNA matching essential genes in the digestive tract of two important agricultural insect pests, the cotton bollworm Helicoverpa armigera (Lepidoptera) [26] and the western corn rootworm, Diabrotica virgifera virgifera (Coleoptera) [23]. In both cases, mortality was achieved after feeding on artificial diet containing dsRNA and GM plants expressing those dsRNAs had increased resistance towards the insects. These works support RNAi as a promising methodology for insect-pest control, making the search for candidate genes to be silenced an important step in control achievement.
RNA-mediated gene silencing as a mechanism was first described in plants as post-transcriptional gene silencing (PTGS) [27,28]. However, the discovery of the interference RNA mechanism (RNAi) in the free-living nematode Caenorhabditis elegans led to the understanding of the core characteristics of RNA-mediated gene silencing [29,30]. RNAi pathway is a natural cell mechanism in which mRNA-complementary dsRNA hybridizes specifically to mRNA leading to its degradation by enzyme complexes. The basic process seems to be conserved in the species studied so far. However, significant differences have been reported concerning the amplification and spread of systemic silencing signal and the silencing effect inheritance [25,31]. Opposite to C. elegans, the RNAi silencing effect in insects is restricted to the site of dsRNA delivery and endures shortly. So far, no gene was reported to be involved in a systemic mechanism for RNAi in insects, although studies have shown RNAi systemic effect in T. castaneum [32–34]. In this context, the sequencing of insect genomes and transcriptomes may provide more information about the genes involved in RNAi silencing pathway [35].
In this work, analysis of more than 500,000 reads obtained by 454-pyrosequencing, assembled in 20,384 contigs is reported. Predicted proteins were compared to known insect genomes: B. mori, T. castaneum and D. melanogaster. Moreover, the analysis of contigs related to core interference RNA mechanism was performed by comparison to the RNAi insect genes. The sequences generated in this work will be a reliable source for candidate genes involved in essential physiological processes to be used in insect control using gene silencing via RNAi. In addition, dsRNA synthesized using A. grandis chitin synthase 1 gene as a template was delivered to cotton boll weevil female adults and managed to trigger chitin synthase 1 silencing.
Materials and Methods
Insects
Eggs, larvae and adult cotton boll weevils were reared in artificial diet according to Monnerat et al [36] at the Laboratório de Bioecologia e Semioquímicos de Insetos of Embrapa Recursos Genéticos e Biotecnologia in Brasília, Brazil. The insects were kept at 26 ± 2 °C, 60 ± 10% relative humidity and 12 h:12 h light:dark. Larvae instars were determined by measuring head capsule width, as described for lepidopterans [37]. Adult sex determination was performed according to Sappington and Spurgeon [38].
RNA purification, cDNA library construction/normalization and pyrosequencing
Total RNA was extracted separately from each insect stage, eggs, larvae (3 instars), pupae and male and female adults using Trizol Reagent (Invitrogen Life Technologies), according to the manufacturer. RNA was treated with RNAse-free DNAse I (Ambion, Invitrogen Life Sciences) at 37 °C for 30 minutes, according to the manufacturer. A pool of 30µg of all insect stages total RNA was sent to synthesize a cDNA library at Eurofins MWG Operon, in Huntsville, AL, USA (http://www.eurofinsdna.com/).
The RNA quality was assessed in a Agilent 2100 Bioanalyzer before cDNA library construction. Full-length, enriched, cDNAs were generated using the SMART PCR cDNA synthesis kit (BD Clontech) following the manufacturer’s protocol. In order to prevent over-representation of the most common transcripts, the resulting double-stranded cDNAs were normalized using the Kamchatka crab duplex-specific nuclease method (Trimmer cDNA normalization kit, Evrogen) [39]. Normalized cDNA was submitted to half-plate run 454 pyrosequencing, GS FLX Titanium technology, according to protocols provided by manufacturer (Roche 454 Life Sciences).
Pre-processing
Pyrosequenced reads were pre-processed using est2ssembly 1.03 pipeline [40]. Contaminant sequences (prokaryotic, viral, mitochondrial sequences) were removed after BLAST analysis. Transcriptome data was deposited in NIH Short Read Archive, with accession number SRA059959.
Assembly, Annotation and Gene Ontology (GO)
Contigs were assembled using MIRA v3.3.0.1 [41]. Homology searches (BLASTX and BLASTN) of unique sequences and functional annotation by GO terms (www.geneontology.org), InterPro entries (InterProScan; http://www.ebi.ac.uk/Tools/pfa/iprscan/), enzyme classification codes (EC) and metabolic pathways (KEGG, Kyoto Encyclopedia of Genes and Genomes; http://www.genome.jp/kegg/) were determined using Blast2GO software suite v2.4.3 (www.blast2go.org). Sequences were submitted to NCBI protein nr databank via BLASTx, with e-value threshold of 10-5. False Discovery Rate (FDR) was used at 0.05% probability level. GO terms were improved with ANNEX tool [42], followed by GOSlim tool available at Blast2GO (goslim_generic.obo) [43]. Combined graphs were constructed at levels 2, 3 and 5 for Biological Process, Cellular Component and Molecular Function categories, respectively. Enzymatic classification codes and KEGG metabolic pathways were generated of direct mapping of GO terms, with their respective ECs. InterPro searches were performed remotely from Blast2GO on InterProEBI server.
A comparison of cotton boll weevil transcriptome to Tribolium castaneum genome annotation was carried out using WEGO tool (Web Gene Ontology Annotation Plotting - http://wego.genomics.org.cn) [44].Venn Diagram was constructed using a free tool found in the Bioinformatics and Evolutionary Genomics Laboratory website, hosted by the University of Gent Plant System Biology Department (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Sequence alignment, SID phylogenetic analysis and in silico analysis of PAZ Domain contigs
Textual and sequence similarity searches were performed in the transcriptome database for genes involved in RNAi mechanism, based on available NCBI Protein Database sequences (http://www.ncbi.nlm.nih.gov/protein).
The amino acid sequences of PAZ domains and SID proteins were obtained by in silico translation using TrEMBL (http://www.expasy.ch/tools/dna.html).
Two largest PAZ Domain-containing contigs, here called A_grandis_454_c1018 and A_grandis_454_c4142 were selected for alignment with PAZ domains of argonautes and dicer-like proteins of other organisms including insects (Figure S1A). All sequences used for alignment contained full PAZ Domains, including A. grandis contigs and were submitted to ClustalW2 Multiple Sequence Alignment (http://www.ebi.ac.uk/Tools/msa/clustalw2/) [45] and edited with Jalview tool (http://www.jalview.org/) [46].
For SID-like protein analysis, a complete gene sequence of A. grandis A_grandis_454_c2889 was translated. Sequence alignment was carried out using complete protein sequences (Figure S1B). Full SID proteins were aligned using Clustal W [47]. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5 [48]. The results of alignments were used for constructing neighbor-joining trees with bootstrap analysis of 10,000 replicates and evolutionary divergence calculated by p-distance method.
Chitin Synthase dsRNA bioassay
Chitin Synthase 1 contig (AntgCHS1) was searched in A. grandis transcriptome using tBLASTx. A specific fragment of 253 bp was chosen using BLOCK-iT™ RNAi Designer (http://rnaidesigner.invitrogen.com/rnaiexpress/). The fragment was amplified with primers 5’ATCACAGGAGCAGCGTTGC and 3’ACACCAACTTATCCAATATC, both containing T7 promoter minimum sequence at 5’ end. PCR product was cloned to pGEMT-easy vector (Promega) and sequenced to verify correct amplification. dsRNA was synthesized with 0,5 µg of PCR product as template, using MEGAscript® T7 High Yield Transcription kit (Invitrogen). AntgCHS1 dsRNA was dissolved in DEPC-treated water and quantified by spectrophotometry.
Female adults aged 48 hours were microinjected with 1 µL of 200 ng AntgCHS1 dsRNA before copulation, using a 10 µL Gastight Luer connection (LT) syringe (1701LT model), with a 51 mm, gauge 26S, point style 4, 12° bevel needle. Each experimental unit consisted of 16 female adults, which, after microinjection were kept in a sieved box with 8 non-injected males, in order to allow the collection of laid eggs. Males were previously marked with ink. The sieved box with insects contained artificial diet [36] and was maintained at 26 ± 2 °C, 60 ± 10% relative humidity and 12/12-hour day/night photoperiod. Control was performed by microinjection of E. coli gus gene dsRNA, produced as previously described. Analyzed phenotypic parameters were oviposition, egg viability, and adult mortality. Eggs laid by microinjected females were mechanically pierced and kept in artificial diet for 7 days. Neonate larvae development and phenotypes were assessed. Data of three bioassays were applied to variance analysis and Tukey’s multiple comparisons test at 5% level of significance.
Results and Discussion
Sequencing, Assembly and Annotation
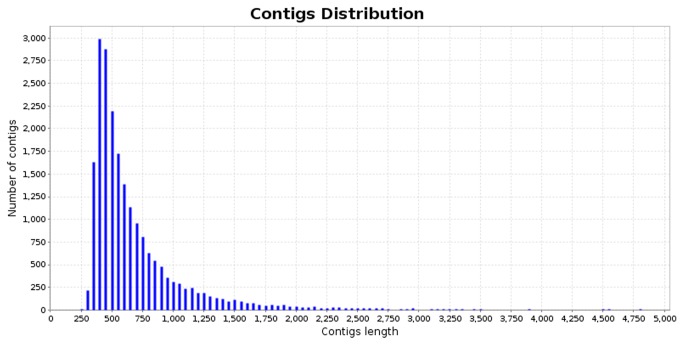
A cDNA library was constructed after pooling RNA extracted from boll weevil eggs, three larval instars, pupae, male and female adults. cDNA-normalized library 454 runs generated a total of 576,297 ESTs (Table 1). The minimal quality standards used in pre-processing provided 310,182 reads, with average read length of 379 bp. These data were deposited in NIH Short Read Archive, with accession number SRA059959. After assembly, 20,384 contigs with average length of 676bp were obtained, with an average depth of coverage of 9.58 sequences per nucleotide position. Of these, most contigs have length ranging from 300 to 750 bp (Figure 1).
Table 1. Summary of Anthonomus grandis transcriptome sequencing assembly and annotation.
| Number of reads before pre-processing | 576,297 |
| Number of bases before pre-processing | 179,676,724 |
| Average read length before pre-processing (bp) | 379 |
| Number of reads after pre-processing | 310,182 |
| Number of bases after pre-processing | 119,094,383 |
| Average read length after pre-processing (bp) | 383 |
| Number of contigs | 20,384 |
| Number of bases in contigs | 13,780,583 |
| Average contig length (bp) | 676 |
| Min. contig length (bp) | 201 |
| Max. contig length (bp) | 4,847 |
| Average read coverage per contig | 9,58 |
| % contigs with at least 1 IPR | 70 |
| Contigs with at least 1 blast hit against nr | 10,621 |
Figure 1. Contigs length distribution showing the major number ranging from 350 to 1000 pb.
The average contig length was 676pb.
Similarity searches and gene ontology analysis
After read assembly, contigs were submitted to BLASTx similarity search against NCBI non-redundant protein database (nr) to assess their putative function. Around 10,600 contigs showed at least one hit against nr (Table 1). Of these, 84.9% showed significant blast matches at a cutoff e-value ≤ 10-3 (Figure S2). Contigs with e-value = 0 were represented at the end of the figure, and correspond to 2.5% of the total number of contigs.
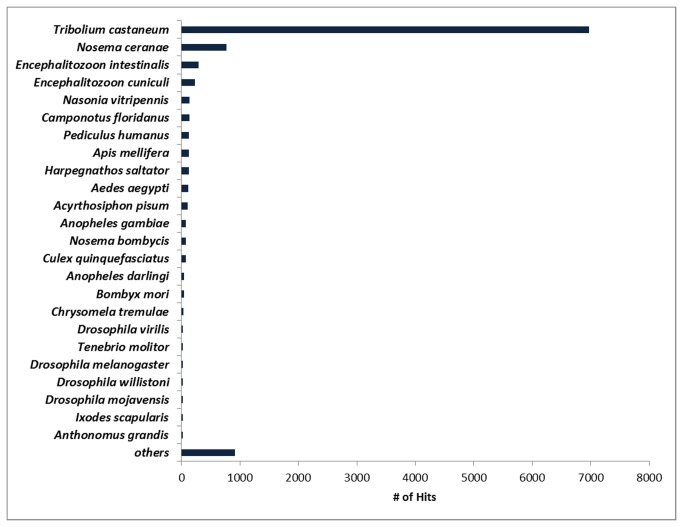
Figure 2 shows the top-hit species after BLASTx similarity search. As expected, 65.7% of the contigs were similar to T. castaneum sequences. T. castaneum (red flour beetle) is the most important coleopteran of Tenebrionidae family because it attacks stored grain products and is responsible for great loss and damage. Until now, it is the only coleopteran with a fully sequenced genome available [49], which explains the far greater number of contigs of A. grandis with similarity to T. castaneum sequences. The three top matching species after Tribolium are fungi. Insect transcriptome pyrosequencing reports show a number of contigs of the Phylum Microsporidia. Nosema is a genus of microsporidian known to parasite a great number of arthropods. Insect orders parasited include Orthoptera, Lepidoptera, Diptera, Hymenoptera and Coleoptera. It is a very common contamination in boll weevil colony rearing and is found in the insect midgut [50]. The most studied species so far, with a whole sequenced genome, is Nosema ceranae, which cause a disease in honey bees and thus great loss to apiculture. The microsporidian genus Encephalitozoon is also described associated in symbiosis to insects [51,52]. Very important in human health, seven genome-sequencing projects of three Encephalitozoon species are deposited in the NCBI Genome Bank. Our data suggest that our insect colony was possibly infected by those microsporidians and some of their ESTs sequenced and well annotated due to the great number of available sequences on databases.
Figure 2. Species distribution of top BLASTx matches of A. grandis contigs.
A great number of contigs matched insect genes, mainly another coleopteran, T. castaneum. E-value cutoff is 1x10-3.
The most part of ESTs was similar to insect sequences. Besides the coleopteran T. castaneum, the other insect species with full genome sequences, although phylogenetically distant, are distributed into the orders Hymenoptera (Nasonia vitripennis, Camponotus floridanus, Apis mellifera, Harpegnathos saltator), Phthiraptera (Pediculus humanus), Diptera (Aedes aegypti, Anopheles gambiae, Culex quinquefasciatus, Anopheles darlingi, Drosophila virilis, Drosophila willistoni, D. melanogaster, Drosophila mojavensis), Hemiptera (Acyrthosiphon pisum), and Lepidoptera (B. mori). The coleopterans Tenebrio molitor and Chrysomela tremulae also were among the top-hit species, but with a low number of matched contigs, probably because they do not have their genomes sequenced yet. This may also explain why A. grandis has a low number of matched sequences.
The A. grandis transcriptome was GO-annotated based on matches to Interpro proteins. In order to group the proteins with associated GO terms, the top level terms for each GO category "Molecular function", "Biological Process" and "Cellular component" were recorded at the different match levels. The dominant terms for Molecular function are clearly transporter activity and binding, while the dominant term for Biological process is pigmentation. Within Cellular component the dominant terms are evenly divided between organelle, cell part and organelle part (Figure S3A, B and C).
A more detailed classification of the contigs function can be obtained from the top 35 InterPro entries (Table 2). The most abundant entry is NAD(P)-binding domain (IPR016040). Chaperones, nucleic acid binding and oxidative stress-related domains constitute the most part of InterPro entries, in accordance to the grouped GO top terms (Figure S3A, B and C).
Table 2. Main protein families found in cotton boll weevil transcriptome.
| InterPro Entry Accession | # of Contigs | InterPro Entry Name |
|---|---|---|
| IPR016040 | 154 | NAD(P)-binding domain |
| IPR011009 | 145 | Protein kinase-like domain |
| IPR016196 | 116 | Major facilitator superfamily domain, general substrate transporter |
| IPR011046 | 110 | WD40 repeat-like-containing domain |
| IPR015943 | 101 | WD40/YVTN repeat-like-containing domain |
| IPR015880 | 94 | Zinc finger, C2H2-like |
| IPR012677 | 88 | Nucleotide-binding, alpha-beta plait |
| IPR016024 | 84 | Armadillo-type fold |
| IPR000504 | 83 | RNA recognition motif domain |
| IPR001680 | 79 | WD40 repeat |
| IPR012336 | 77 | Thioredoxin-like fold |
| IPR007087 | 73 | Zinc finger, C2H2 |
| IPR017853 | 73 | Glycoside hydrolase, superfamily |
| IPR002198 | 67 | Short-chain dehydrogenase/reductase SDR |
| IPR013781 | 67 | Glycoside hydrolase, subgroup, catalytic domain |
| IPR009003 | 66 | Peptidase cysteine/serine, trypsin-like |
| IPR001254 | 65 | Peptidase S1/S6, chymotrypsin/Hap |
| IPR011992 | 59 | EF-hand-like domain |
| IPR001650 | 55 | Helicase, C-terminal |
| IPR000618 | 54 | Insect cuticle protein |
| IPR001128 | 54 | Cytochrome P450 |
| IPR001611 | 49 | Leucine-rich repeat |
| IPR002290 | 45 | Serine/threonine- / dual-specificity protein kinase, catalytic domain |
| IPR002018 | 44 | Carboxylesterase, type B |
| IPR009057 | 44 | Homeodomain-like |
| IPR011989 | 44 | Armadillo-like helical |
| IPR011990 | 44 | Tetratricopeptide-like helical |
| IPR016027 | 44 | Nucleic acid-binding, OB-fold-like |
| IPR015424 | 42 | Pyridoxal phosphate-dependent transferase, major domain |
| IPR003959 | 41 | ATPase, AAA-type, core |
| IPR012340 | 41 | Nucleic acid-binding, OB-fold |
| IPR002557 | 40 | Chitin binding domain |
| IPR009072 | 40 | Histone-fold |
| IPR011701 | 40 | Major facilitator superfamily |
| IPR001353 | 39 | Proteasome, subunit alpha/beta |
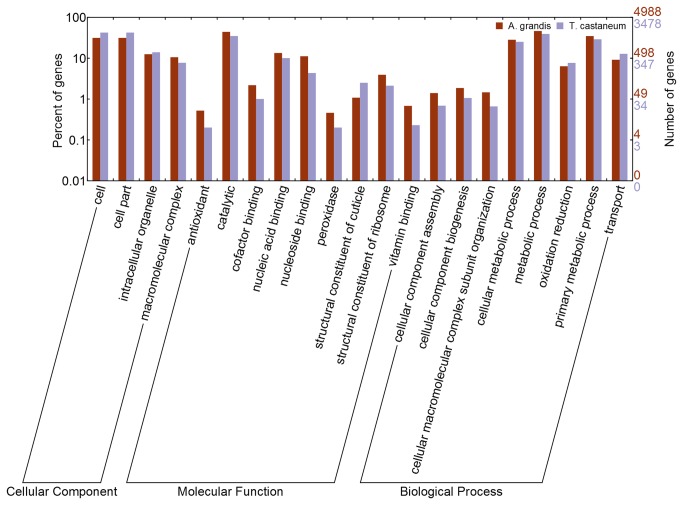
We used WEGO [44] for visualizing and comparing our GO annotation to the T. castaneum genome annotation data (Figure 3). Similar number of genes was annotated for the same GO terms in both insects for a determined GO category and no significant differences were shown, which indicates that de novo annotation for A. grandis is comparable to the T. castaneum genome annotation. Hence, we consider that we accomplished the objective of generating a database describing a significant and representative portion of the A. grandis transcriptome.
Figure 3. Comparison of the distribution of GO terms.
The X-axis shows subgroups of cellular component, molecular functions and biological process from GO. Distribution of GO terms of gene families of T. castaneum and A. grandis are compared. The Y-axis shows the percentage (left) and the number of genes (right) of the matched Pfam entries.
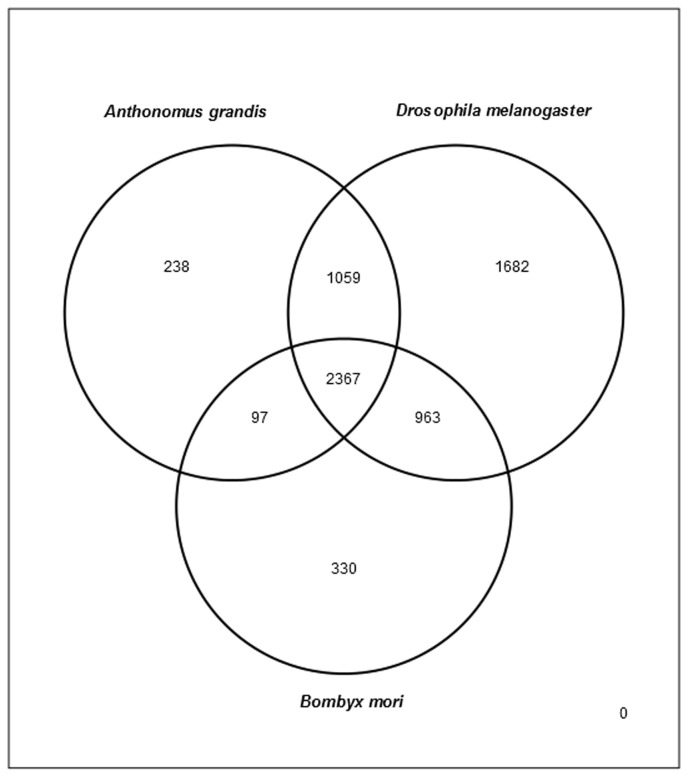
We performed a comparison of the A. grandis 454 Pfam entries to D. melanogaster and B. mori Pfam transcript sects from Flybase [53] and Silkbase [54] (with tBLASTx, e < 10-3) in order to establish a simplified genetic overlap between these species. The low number of A. grandis sequences, which do not match either D. melanogaster or B. mori (Figure 4) is probably due to the sum of new unique genes, poorly conserved genes, and erroneously sequenced reads. We noticed that the protein family similarity is higher to Drosophila (Diptera) than to Bombyx (Lepidoptera). This is significant because the number of sequence data in plant-insect pest interaction is greater for Lepidoptera than for Diptera, which normally lead to a probably erroneous biased search for ortholog sequences for coleopterans in lepidopteran databases.
Figure 4. Venn diagram of the number of contigs from A. grandis which show IPR matches to D. melanogaster and/or B. mori.
Numbers are unique Butterflybase and Flybase IPR results. The number of similar protein families between A.grandis and D. melanogaster is higher than A.grandis and B. mori.
Proteins involved in RNA interference mechanism
The mechanisms of RNAi seem to be conserved among species, despite the previously described differences regarding signal amplification, systemic effect and inheritance [32]. In insects, except dipterans, dsRNA uptake is carried out by SID-1. Once inside the cell, dsRNA is cleaved in small RNAs (siRNAs) by Dicers. siRNAs are recognized by the RNA-induced silencing complex (RISC), which contain argonaute proteins. The siRNAs hybridize with specific mRNAs and the duplex siRNA-target mRNA is then degraded. We have found several contigs of genes coding for proteins involved in RNAi mechanisms (Figure 5). Most proteins sequenced belonged to Argonaute, Dicer and Helicase families, involved in dsRNA cleavage and endonuclease activity. The number of contigs found for each gene class is indicated.
Figure 5. Genes involved in RNAi mechanism found in A. grandis transcriptome.
The comparison with genes of C. elegans, T. castaneum, and D. melanogaster suggested that RNAi mechanism is well conserved in insects (A, B, C, D), including lack of amplification (E). No gene involved in dsRNA degradation was found (F). The number of contigs found in A. grandis transcriptome for each gene class is shown.
Based on the contigs found, RNAi mechanism in A. grandis seems to be similar to other insects in the steps of the process like dsRNA cleavage, dsRNA binding and Argonaute activity (Figure 5B, C, D), but differs of dipterans in dsRNA uptake (Figure 5A). No gene involved in dsRNA degradation was found (Figure 5F). The contigs found best matched insect genes, mainly from dipteran and coleopteran species (Table S1).
Two sid-1 contigs (A_grandis_454_c14864, A_grandis_454_rep_c2889, 709bp and 1918bp, respectively), gene that codes for the membrane protein responsible for dsRNA uptaking and spreading through the tissues, were found. The top species BLASTx hit for these two contigs was T. castaneum, which has three sid-1 paralogs in its genome. Both contigs have above 60% identity and e-value < 5x10-31. Those contig sequences do not overlap, and probably are paralog genes. Their best BLASTx hits are T. castaneum sid-1A and sid-1C, respectively.
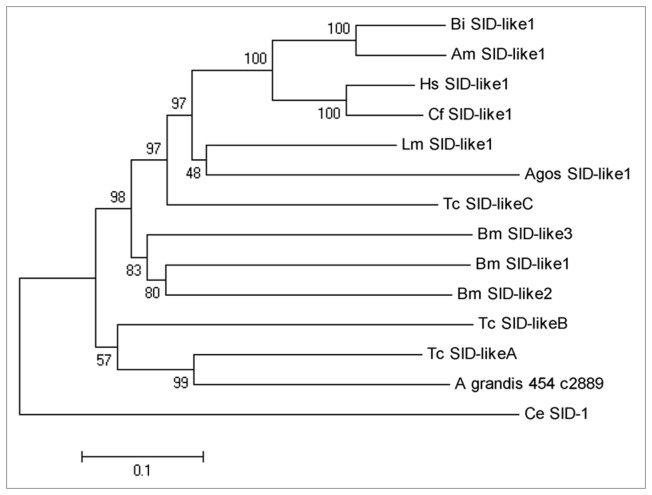
We used the predicted protein from contig A_grandis_454_c2889 for phylogenetic analysis because it contains the complete ORF for sid-1. A distance/neighbor-joining dendrogram for the SID proteins grouped the A_grandis_454_c2889 contig with SID-like A and SID-like B from T. castaneum (Figure 6). SID-like C from T. castaneum is closer to hemipteran A. gossypii and grouped in the branch that have homopteran and mainly hymenopteran insects. Probably, the contig A_grandis_454_c14864 that has as BLASTx best hit sid-1C of T. castaneum, could group in the same branch, although we need full gene sequence to confirm it. An evaluation of available genomes shows that the number of sid-1 gene copies varies among insects. Dipterans have no sid-1 genes, and hemipterans, hymenopterans, orthopterans and phthirapterans have just one sid-1 [35]. Among lepidopterans this number are even more variable: while B.mori has three, Spodoptera exigua has only one [25,35]. As previously described for other insects, no sid-2 ortholog gene, which is present in nematodes, was found for A. grandis.
Figure 6. Distance neighbor-joining tree showing the phylogeny of a SID-like contig of A. grandis (A_grandis_454_c2889) and SID-like proteins of the insects T. castaneum, B. impatiens, A. mellifera, L. migratoria, B. mori, A. gossypii, H. saltator, Camponotus floridanus.
The percentage of percentage of bootstrap confidence values is shown at the nodes.
No ortholog gene for RNA-dependent RNA polymerase (RdRP), the enzyme that amplifies RNAi signal in nematodes, was found (Figure 5E). Recent studies performed and patented by our group showed that delivery of dsRNA by microinjection was capable of trigger silencing of laccase2 [55] and chitin synthase 2 [56] genes in A. grandis. Since the morphological effects were observed far from the local of microinjection, this corroborates the already proposed hypothesis that when RNAi signal amplification occurs in insects, mainly in coleopterans, it may be mediated by other mechanism [57].
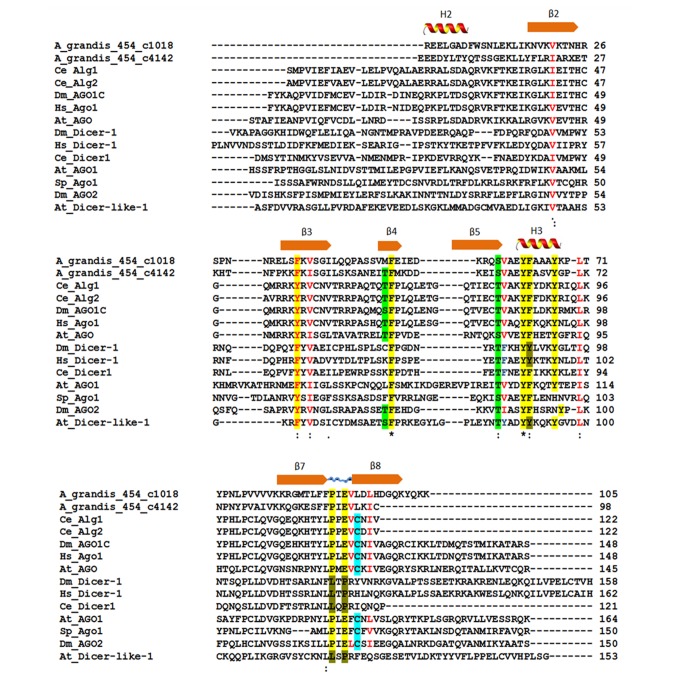
In order to evaluate a conserved domain in a protein involved in RNAi mechanism, we performed an alignment of the PAZ domains of two contigs from the boll weevil transcriptome (A_grandis_454_c1018 and A_grandis_454_c4142) with dicers and argonautes of 5 different species: (Figure 7): D. melanogaster (Dm_Dicer-1, Dm_AGO1C, Dm_AGO2), C. elegans (Ce_Dicer1, Ce_Alg1, Ce_Alg2), Homo sapiens (Hs_Dicer-1, Hs_Ago1), Arabidopsis thaliana (At_Dicer-like-1, At_AGO, At_AGO1) e Schizosaccharomyces pombe (Sp_AGO1). PAZ is a double-stranded-RNA-binding domain present in all argonautes and dicers [31,58]. Conserved residues in dicers and argonautes are also present in A. grandis contigs, which can validate in transcriptome assembly. These residues are normally located on the domain surface and at only one side of the RNA-binding proteins [59]. In figure 7, the highlighted residues are responsible for the stabilization of the dsRNA-binding region, forming seven β structures and a α-helix. A subdomain featuring aromatic residues (in yellow) keep the domain folding which is similar to an OB-fold (OB – Oligonucleotide/oligosaccharide Binding fold), known to bind single-stranded DNA unspecifically [60,61]. Along with a cysteine residue (blue), preceded by a proline and a glutamate (yellow), some invariant residues (red) create a hydrophobic subdomain that interacts with RNA. Differential residues between PAZ domains of dicers and argonautes suggest that the cotton boll weevil contigs belong to the latter family (in brown), although experimental approaches are necessary to confirm it.
Figure 7. Comparison of dicer and argonaute PAZ domains.
Two cotton boll weevil contigs were aligned to five species sequences: D. melanogaster (Dm_Dicer-1, Dm_AGO1C, Dm_AGO2), C. elegans (Ce_Dicer1, Ce_Alg1, Ce_Alg2), Homo sapiens (Hs_Dicer-1, Hs_Ago1), A. thaliana (At_Dicer-like-1, At_AGO, At_AGO1) and Schizosaccharomyces pombe (Sp_AGO1). The sequence IDs are the same found in the NCBI Protein Database. Secondary structures within the domain are indicated as α-helices and β structures. The highlighted residues are responsible for the stabilization of the dsRNA-binding region. In yellow, a subdomain of aromatic residues. Along with a cysteine residue (blue), preceded by a proline and a glutamate (yellow), some invariant residues (red) create a hydrophobic subdomain that interacts with RNA. Residues that differ in dicer and argonaute PAZ domains are shown in brown.
Effect of parental AntgCHS1 dsRNA on eggs and neonate larvae
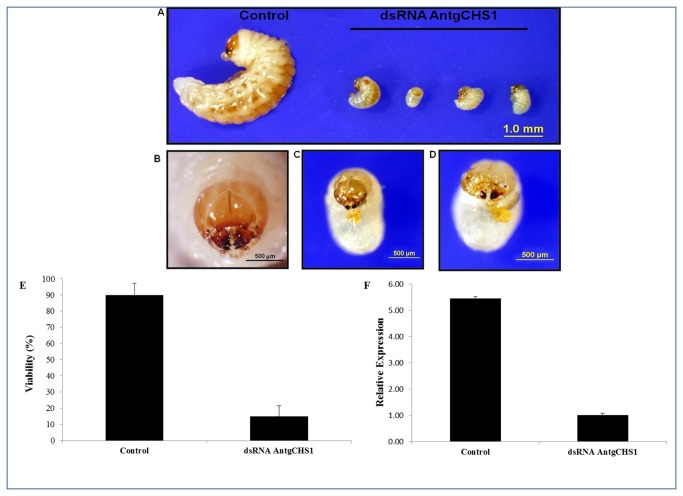
Chitin synthase (EC 2.4.1.16) is the final enzyme of the chitin synthesis pathway which polymerizes chitin by promoting covalent bonds between activated UDP-N-acetylglucosamine monomers [62]. Gene silencing reports have showed the importance of chitin biosynthesis for insect cuticle formation [63,64]. A chitin synthase contig was found in A. grandis transcriptome and here called AntgCHS1, corresponding to chitin synthase 1, enzyme described to trigger chitin polymerization in insect cuticle [62,65]. In order to evaluate the effect of RNAi gene silencing on A. grandis, GUS dsRNA and AntgCHS1 dsRNA were synthesised and delivered to female adults by microinjection before copulation. No effect was phenotipically observed in the microinjected females. AntgCHS1 dsRNA microinjection caused no effect in female survival. After copulation, the number of laid eggs was not different between treatments, but viability, measured as the average number of eggs which hatched and generated well-formed larvae, was reduced 84% for eggs laid by AntgCHS1 dsRNA (Figure 8E). Interestingly, embryo formation and normal movement inside the same eggs were observed, suggesting that larvae were formed but could not eclose. So mechanical perforation of egg shell was performed and larvae transferred to artificial diet and observed for seven days. Larvae from GUS dsRNA-microinjected females developed normally, while larvae from AntgCHS1 dsRNA-treated females failed to develop and died (Figure 8A). This can be explained by the observed head capsule and mandibule malformation which must have hampered diet feeding as well the previously described difficulty of tearing the egg shell and eclosing (Figure 8B, C, D). Previous studies have already reported the incapacity of egg shell rupture by larvae in which chitin synthesis was compromised [66]. Mutations in D. melanogaster chs 1 gene, formerly called kkv, caused the embryos to develop normally, but to fail in eclode from the eggs. When the vitelline membrane in these mutant eggs was punctured by mechanic pressure, embryos were alive and more stretched than wild-type embryos. This phenotype, called blimp, was explained by the failure of epidermal cells in synthesize the cuticle correctly. Loss of functionality in chitin synthases, either by mutation or by the use of synthetic inhibitors, like benzoylphenyl urea (BPU) can produce the same results [67–69].
Figure 8. Effect of AntgCHS1 on A. grandis on oviposition.
Larvae that emerged from eggs laid by females previously microinjected with 200 ng of either GUS (control) or AntgCHS1 dsRNA (A). After egg hatching, larvae were fed in artificial diet for 7 days. Details of head capsule show malformations in AntgCHS1 dsRNA-treated larvae (C and D) when compared to control (B). The viability was reduced (E) and as well as the number of transcripts of AntgCHS1 (F) in eggs laid by females previously microinjected with AntgCHS1 dsRNA.
In addition, eggs laid by microinjected females after copulation were used to evaluate the number of AntgCHS1 gene transcripts. The microinjection of 200 ng of AntgCHS1 dsRNA in adult females resulted in a 5,5-fold reduction of AntgCHS1 gene transcripts in eggs when compared to control, indicating that RNAi effect was transferred to the next generation (Figure 8F). These results confirm that synthesis of chitin in insect epidermis is affected in A. grandis after AntgCHS1 dsRNA delivery. Parental RNAi effect transferred to offspring was also reported for T. castaneum genes [32,34]. As discussed before, these facts also support the theory of at least one unknown mechanism of RNAi signal amplification, which is different from nematodes and plants, since insects do not have RdRP genes in their genomes.
Conclusions
Here it is described the analysis of a new database of cotton boll weevil (A. grandis) nucleotide sequences obtained by pyrosequencing of the insect transcriptome. It is the largest number of sequences provided for this insect pest so far. These results provide a significant molecular biology dataset, which can be used, as an example, for molecular prospection in order to validate genes to be used in insect control. The silencing of a chitin synthase gene in larvae emerged from eggs laid by dsRNA-microinjected females proved that not only RNAi machinery is able to trigger RNAi silencing in A. grandis, but also to transfer its effect to the next generation. Since the main goal here was to generate and analyze data in silico, other experiments of gene expression quantitation, silencing via RNAi and gene sequencing in specific insect stages or submitted to certain conditions must be carried out. These experiments will allow the characterization of processes, either to understand cotton boll weevil biology or to assess gene candidates for development of insect control biotechnological tools.
Supporting Information
Orthologous genes used in PAZ Domain alignment (A) and SID-1 phylogenetic Analysis (B). Two largest cotton boll weevil PAZ Domain-containing contigs were selected for alignment with PAZ domains of argonautes and dicer-like proteins of other organisms including insects. For SID-like protein phylogenetic analysis, a cotton boll weevil complete gene sequence was translated and aligned to complete protein sequences.
(TIF)
E-value for the top BLASTx hits. Sequences with e-value equal to 0 are represented in a peak at right. 84.9% of the contigs showed significant blast matches at a cutoff e-value ≤ 10-3.
(TIF)
Gene ontology (GO) categories for A. grandis transcriptome. The terms were classified on level 2, 3 and 5 in the (A) Biological Process, (B) Cellular Component and (C) Molecular Function, respectively. The dominant terms for Molecular function are transporter activity and binding, while the dominant term for Biological process is pigmentation. Within Cellular component the dominant terms are evenly divided between organelle, cell part and organelle part. The percentage of contigs in each GO term is shown.
(TIF)
A. grandis contigs found in the transcriptome corresponding to RNAi insect genes. RNAi mechanism in A. grandis seems to be similar to other insects in the steps of the process like dsRNA cleavage, dsRNA binding and Argonaute activity, but differs of dipterans in dsRNA uptake. No gene involved in dsRNA degradation was found.
(XLSX)
Acknowledgments
The authors thank Isabela Grisi for assisting with the collection and maintenance of A. grandis populations. The authors also thank Dr. Walter Terra for support on insect microinjection methodology.
Funding Statement
This work was supported by CAPES (http://www.capes.gov.br/), EMBRAPA (http://www.embrapa.br/), and CNPq (http://www.cnpq.br/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Consortium ikI (2012) 5,000 Insect Genome Project. Arthropod Genomes [Google Scholar]
- 2. Legrand S, Valot N, Nicolé F, Moja S, Baudino S et al. (2010) One-step identification of conserved miRNAs, their targets, potential transcription factors and effector genes of complete secondary metabolism pathways after 454 pyrosequencing of calyx cDNAs from the Labiate Salvia sclarea L. Gene 450: 55-62. doi: 10.1016/j.gene.2009.10.004. PubMed: 19840835. [DOI] [PubMed] [Google Scholar]
- 3. Shin H, Hirst M, Bainbridge MN, Magrini V, Mardis E et al. (2008) Transcriptome analysis for Caenorhabditis elegans based on novel expressed sequence tags. BMC Biol 6: 30. doi: 10.1186/1741-7007-6-30. PubMed: 18611272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mardis ER (2008) Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet 9: 387-402. doi: 10.1146/annurev.genom.9.081307.164359. PubMed: 18576944. [DOI] [PubMed] [Google Scholar]
- 5. Ahmadian A, Ehn M, Hober S (2006) Pyrosequencing: history, biochemistry and future. Clin Chim Acta 363: 83-94. doi: 10.1016/j.cccn.2005.04.038. PubMed: 16165119. [DOI] [PubMed] [Google Scholar]
- 6. Li S, Mead EA, Liang S, Tu Z (2009) Direct sequencing and expression analysis of a large number of miRNAs in Aedes aegypti and a multi-species survey of novel mosquito miRNAs. BMC Genomics 10: 581. doi: 10.1186/1471-2164-10-581. PubMed: 19961592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hahn DA, Ragland GJ, Shoemaker DD, Denlinger DL (2009) Gene discovery using massively parallel pyrosequencing to develop ESTs for the flesh fly Sarcophaga crassipalpis. BMC Genomics 10: 234. doi: 10.1186/1471-2164-10-234. PubMed: 19454017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gregory R, Darby AC, Irving H, Coulibaly MB, Hughes M et al. (2011) A de novo expression profiling of Anopheles funestus, malaria vector in Africa, using 454 pyrosequencing. PLOS ONE 6: e17418. doi: 10.1371/journal.pone.0017418. PubMed: 21364769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang F, Guo H, Zheng H, Zhou T, Zhou Y et al. (2010) Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV). BMC Genomics 11: 303. doi: 10.1186/1471-2164-11-303. PubMed: 20462456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou Z, Najar F, Wang Y, Roe B, Jiang H (2008) Pyrosequence analysis of expressed sequence tags for Manduca sexta hemolymph proteins involved in immune responses. Insect Biochem Mol Biol 38: 677-682. doi: 10.1016/j.ibmb.2008.03.009. PubMed: 18510979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pauchet Y, Wilkinson P, van Munster M, Augustin S, Pauron D et al. (2009) Pyrosequencing of the midgut transcriptome of the poplar leaf beetle Chrysomela tremulae reveals new gene families in Coleoptera. Insect Biochem Mol Biol 39: 403-413. doi: 10.1016/j.ibmb.2009.04.001. PubMed: 19364528. [DOI] [PubMed] [Google Scholar]
- 12. Pauchet Y, Wilkinson P, Vogel H, Nelson DR, Reynolds SE et al. (2010) Pyrosequencing the Manduca sexta larval midgut transcriptome: messages for digestion, detoxification and defence. Insect Mol Biol 19: 61-75. doi: 10.1111/j.1365-2583.2009.00936.x. PubMed: 19909380. [DOI] [PubMed] [Google Scholar]
- 13. Pauchet Y, Wilkinson P, Chauhan R, Ffrench-Constant RH (2010) Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLOS ONE 5: e15635. doi: 10.1371/journal.pone.0015635. PubMed: 21179425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zagrobelny M, Scheibye-Alsing K, Jensen NB, Møller BL, Gorodkin J et al. (2009) 454 pyrosequencing based transcriptome analysis of Zygaena filipendulae with focus on genes involved in biosynthesis of cyanogenic glucosides. BMC Genomics 10: 574. doi: 10.1186/1471-2164-10-574. PubMed: 19954531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karatolos N, Pauchet Y, Wilkinson P, Chauhan R, Denholm I et al. (2011) Pyrosequencing the transcriptome of the greenhouse whitefly, Trialeurodes vaporariorum reveals multiple transcripts encoding insecticide targets and detoxifying enzymes. BMC Genomics 12: 56. doi: 10.1186/1471-2164-12-56. PubMed: 21261962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong CN, Ng P, Douglas AE (2011) Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol 13: 1889-1900. doi: 10.1111/j.1462-2920.2011.02511.x. PubMed: 21631690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahais V, Gayral P, Tsagkogeorga G, Melo-Ferreira J, Ballenghien M et al. (2012) Reference-free transcriptome assembly in non-model animals from next-generation sequencing data. Mol Ecol Resour 12: 834-845. doi: 10.1111/j.1755-0998.2012.03148.x. PubMed: 22540679. [DOI] [PubMed] [Google Scholar]
- 18. Keeling CI, Henderson H, Li M, Yuen M, Clark EL et al. (2012) Transcriptome and full-length cDNA resources for the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major insect pest of pine forests. Insect Biochem Mol Biol 42: 525-536. doi: 10.1016/j.ibmb.2012.03.010. PubMed: 22516182. [DOI] [PubMed] [Google Scholar]
- 19. Vera JC, Wheat CW, Fescemyer HW, Frilander MJ, Crawford DL et al. (2008) Rapid transcriptome characterization for a nonmodel organism using 454 pyrosequencing. Mol Ecol 17: 1636-1647. doi: 10.1111/j.1365-294X.2008.03666.x. PubMed: 18266620. [DOI] [PubMed] [Google Scholar]
- 20. America NCCo (2009) Pest Management; —— Boll Weevil Eradication Program. In: (2009), editor. US and Northern Mexico. 2009 ed. Memphis, TN, USA: National Cotton Council of America; . pp. Cotton Pest Management - Boll Weevil Eradication Program. [Google Scholar]
- 21. Freire EC (2011) Algodão no Cerrado. Brasília - DF: ABRAPA - Associação Brasileira dos Produtores de Algodão.
- 22. James C (2011) Global Status of Commercialized Biotech/GM Crops. ISAAA Brief: 2011: 43. [Google Scholar]
- 23. Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P et al. (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25: 1322-1326. doi: 10.1038/nbt1359. PubMed: 17982443. [DOI] [PubMed] [Google Scholar]
- 24. Rangasamy M, Siegfried BD (2012) Validation of RNA interference in western corn rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) adults. Pest Manag Sci 68: 587-591. doi: 10.1002/ps.2301. PubMed: 22500293. [DOI] [PubMed] [Google Scholar]
- 25. Price DR, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotechnol 26: 393-400. doi: 10.1016/j.tibtech.2008.04.004. PubMed: 18501983. [DOI] [PubMed] [Google Scholar]
- 26. Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY et al. (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25: 1307-1313. doi: 10.1038/nbt1352. PubMed: 17982444. [DOI] [PubMed] [Google Scholar]
- 27. Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol Biol 31: 957-973. doi: 10.1007/BF00040715. PubMed: 8843939. [DOI] [PubMed] [Google Scholar]
- 28. Waterhouse PM, Graham MW, Wang MB (1998) Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense. RNA - Proc Natl Acad Sci U S A 95: 13959-13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fire A (1999) RNA-triggered gene silencing. Trends Genet 15: 358-363. doi: 10.1016/S0168-9525(99)01818-1. PubMed: 10461204. [DOI] [PubMed] [Google Scholar]
- 30. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE et al. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806-811. doi: 10.1038/35888. PubMed: 9486653. [DOI] [PubMed] [Google Scholar]
- 31. Sontheimer EJ (2005) Assembly and function of RNA silencing complexes. Nat Rev Mol Cell Biol 6: 127-138. doi: 10.1038/nrm1568. PubMed: 15654322. [DOI] [PubMed] [Google Scholar]
- 32. Bucher G, Scholten J, Klingler M (2002) Parental RNAi in Tribolium (Coleoptera). Curr Biol 12: R85-R86. doi: 10.1016/S0960-9822(01)00651-0. PubMed: 11839285. [DOI] [PubMed] [Google Scholar]
- 33. Posnien N, Schinko J, Grossmann D, Shippy TD, Konopova B et al. (2009) RNAi in the red flour beetle (Tribolium). Cold Spring Harb Protoc 2009: pdb prot5256. [DOI] [PubMed]
- 34. Tomoyasu Y, Denell RE (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol 214: 575-578. doi: 10.1007/s00427-004-0434-0. PubMed: 15365833. [DOI] [PubMed] [Google Scholar]
- 35. Tomoyasu Y, Miller SC, Tomita S, Schoppmeier M, Grossmann D et al. (2008) Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Biol 9: R10. doi: 10.1186/gb-2008-9-s2-s10. PubMed: 18201385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monnerat RG, Dias SC, Oliveira-Neto OB, Nobre SD, Silva-Werneck JO et al. (2000) Criação massal do bicudo do algodoeiro Anthonomus grandis em laboratório. Comunicado Tecnico - Embrapa Recursos Genéticos e Biotecnologia 46: 4. [Google Scholar]
- 37. Calvo D, Molina JM (2008) Head Capsule Width and Instar Determination for Larvae of Streblote panda (Lepidoptera: Lasiocampidae). Annals of the Entomological Society of America 101: 881-886. Available online at: doi:10.1603/0013-8746(2008)101[881:HCWAID]2.0.CO;2 [Google Scholar]
- 38. Sappington TW, Spurgeon DW (2000) Preferred Technique for Adult Sex Determination of the Boll Weevil (Coleoptera: Curculionidae). Annals of the Entomology Society of America 93: 610-615
- 39. Zhulidov PA, Bogdanova EA, Shcheglov AS, Vagner LL, Khaspekov GL et al. (2004) Simple cDNA normalization using kamchatka crab duplex-specific nuclease. Nucleic Acids Res 32: e37. doi: 10.1093/nar/gkh382. PubMed: 14973331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papanicolaou A, Stierli R, Ffrench-Constant RH, Heckel DG (2009) Next generation transcriptomes for next generation genomes using est2assembly. BMC Bioinformatics 10: 447. doi: 10.1186/1471-2105-10-447. PubMed: 20034392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Müller WE et al. (2004) Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res 14: 1147-1159. doi: 10.1101/gr.1917404. PubMed: 15140833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Myhre S, Tveit H, Mollestad T, Laegreid A (2006) Additional gene ontology structure for improved biological reasoning. Bioinformatics 22: 2020-2027. doi: 10.1093/bioinformatics/btl334. PubMed: 16787968. [DOI] [PubMed] [Google Scholar]
- 43. Conesa A, Gotz S (2008) Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics 2008: 619832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ye J, Fang L, Zheng H, Zhang Y, Chen J et al. (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34: W293-W297. doi: 10.1093/nar/gkl031. PubMed: 16845012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947-2948. doi: 10.1093/bioinformatics/btm404. PubMed: 17846036. [DOI] [PubMed] [Google Scholar]
- 46. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189-1191 [DOI] [PMC free article] [PubMed]
- 47. Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673-4680. doi: 10.1093/nar/22.22.4673. PubMed: 7984417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739. doi: 10.1093/molbev/msr121. PubMed: 21546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R et al. (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452: 949-955. doi: 10.1038/nature06784. PubMed: 18362917. [DOI] [PubMed] [Google Scholar]
- 50. Mclaughlin RE (1966) Laboratory Techniques for Rearing Disease-Free Insect Colonies - Elimination of Mattesia Grandis Mclaughlin and Nosema sp from Colonies of Boll Weevils. Journal of Economic Entomology 59: 401-& [Google Scholar]
- 51. Lange CE, Johny S, Baker MD, Whitman DW, Solter LF (2009) A new Encephalitozoon species (Microsporidia) isolated from the lubber grasshopper, Romalea microptera (Beauvois) (Orthoptera: Romaleidae). J Parasitol 95: 976-986. doi: 10.1645/GE-1923.1. PubMed: 20050002. [DOI] [PubMed] [Google Scholar]
- 52. Agnew P, Becnel JJ, Ebert D, Michalakis Y (2003) Symbiosis of Microsporidia and Insects. In: Bourtzis K, Miller TA. Insect symbiosis. Boca Raton, FL: CRC; pp. 145-163. [Google Scholar]
- 53. McQuilton P, St Pierre SE, Thurmond J (2012) FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res 40: D706-D714. doi: 10.1093/nar/gkr1030. PubMed: 22127867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mita K, Morimyo M, Okano K, Koike Y, Nohata J et al. (2003) The construction of an EST database for Bombyx mori and its application. Proc Natl Acad Sci U S A 100: 14121-14126. doi: 10.1073/pnas.2234984100. PubMed: 14614147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grossi-de-Sa MF, Firmino AAP, Silva MCM, Martins-de-Sa D, Coelho RR, et al. (2012) Método e composições para controle genético de insetos-praga em plantas de algodão através do silenciamento de genes da família da Lacase. In: INPI Brazil. [Google Scholar]
- 56. Grossi-de-Sa MF, Macedo LLP, Silva MCM, Firmino AAP, Coelho RR, et al. (2012) Método e composições para controle genético de insetos-praga em plantas de algodão através do silenciamento de genes de quitina sintases. In: INPI . [Google Scholar]
- 57. Gatehouse JA (2008) Biotechnological prospects for engineering insect-resistant plants. Plant Physiol 146: 881-887. doi: 10.1104/pp.107.111096. PubMed: 18316644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Q, Paroo Z (2010) Biochemical principles of small RNA pathways. Annu Rev Biochem 79: 295-319. doi: 10.1146/annurev.biochem.052208.151733. PubMed: 20205586. [DOI] [PubMed] [Google Scholar]
- 59. Song JJ, Liu J, Tolia NH, Schneiderman J, Smith SK et al. (2003) The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat Struct Biol 10: 1026-1032. doi: 10.1038/nsb1016. PubMed: 14625589. [DOI] [PubMed] [Google Scholar]
- 60. Murzin AG (1993) OB(oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J 12: 861-867. PubMed: 8458342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suck D (1997) Common fold, common function, common origin? Nat Struct Biol 4: 161-165. doi: 10.1038/nsb0397-161. PubMed: 9164449. [DOI] [PubMed] [Google Scholar]
- 62. Merzendorfer H (2011) The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol 90: 759-769. doi: 10.1016/j.ejcb.2011.04.014. PubMed: 21700357. [DOI] [PubMed] [Google Scholar]
- 63. Arakane Y, Baguinon MC, Jasrapuria S, Chaudhari S, Doyungan A et al. (2011) Both UDP N-acetylglucosamine pyrophosphorylases of Tribolium castaneum are critical for molting, survival and fecundity. Insect Biochem Mol Biol 41: 42-50. doi: 10.1016/j.ibmb.2010.09.011. PubMed: 20920581. [DOI] [PubMed] [Google Scholar]
- 64. Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW (2008) Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 38: 959-962. doi: 10.1016/j.ibmb.2008.07.006. PubMed: 18718535. [DOI] [PubMed] [Google Scholar]
- 65. Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206: 4393-4412. doi: 10.1242/jeb.00709. PubMed: 14610026. [DOI] [PubMed] [Google Scholar]
- 66. Kennerdell JR, Carthew RW (2000) Heritable gene silencing in Drosophila using double-stranded RNA. Nat Biotechnol 18: 896-898. doi: 10.1038/78531. PubMed: 10932163. [DOI] [PubMed] [Google Scholar]
- 67. Wilson TG, Cryan JR (1997) Lufenuron, a chitin-synthesis inhibitor, interrupts development of Drosophila melanogaster. J Exp Zool 278: 37-44. doi: 10.1002/(SICI)1097-010X(19970501)278:1. PubMed: 9136145. [DOI] [PubMed] [Google Scholar]
- 68. Ostrowski S, Dierick HA, Bejsovec A (2002) Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics 161: 171-182. PubMed: 12019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gangishetti U, Breitenbach S, Zander M, Saheb SK, Müller U et al. (2009) Effects of benzoylphenylurea on chitin synthesis and orientation in the cuticle of the Drosophila larva. Eur J Cell Biol 88: 167-180. doi: 10.1016/j.ejcb.2008.09.002. PubMed: 18996617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Orthologous genes used in PAZ Domain alignment (A) and SID-1 phylogenetic Analysis (B). Two largest cotton boll weevil PAZ Domain-containing contigs were selected for alignment with PAZ domains of argonautes and dicer-like proteins of other organisms including insects. For SID-like protein phylogenetic analysis, a cotton boll weevil complete gene sequence was translated and aligned to complete protein sequences.
(TIF)
E-value for the top BLASTx hits. Sequences with e-value equal to 0 are represented in a peak at right. 84.9% of the contigs showed significant blast matches at a cutoff e-value ≤ 10-3.
(TIF)
Gene ontology (GO) categories for A. grandis transcriptome. The terms were classified on level 2, 3 and 5 in the (A) Biological Process, (B) Cellular Component and (C) Molecular Function, respectively. The dominant terms for Molecular function are transporter activity and binding, while the dominant term for Biological process is pigmentation. Within Cellular component the dominant terms are evenly divided between organelle, cell part and organelle part. The percentage of contigs in each GO term is shown.
(TIF)
A. grandis contigs found in the transcriptome corresponding to RNAi insect genes. RNAi mechanism in A. grandis seems to be similar to other insects in the steps of the process like dsRNA cleavage, dsRNA binding and Argonaute activity, but differs of dipterans in dsRNA uptake. No gene involved in dsRNA degradation was found.
(XLSX)