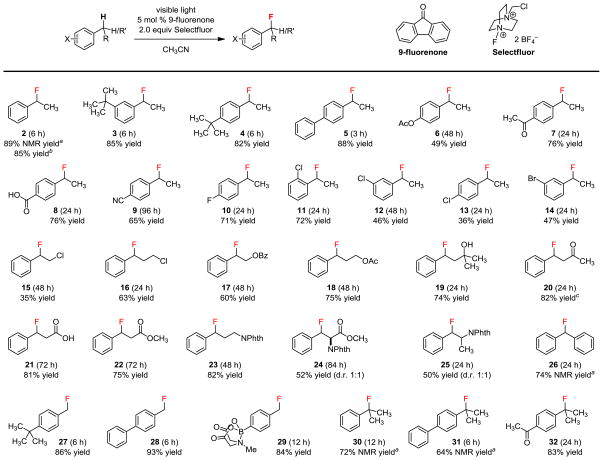
Figure 2.
Scope of the benzylic monofluorination. Conditions: 0.2 mmol substrate, 5 mol % 9-fluorenone, 2.0 equiv Selectfluor, 2.5 mL acetonitrile, CFL irradiation, 27 °C, isolated yield. aDetermined by 19F NMR using C6H5F as an external standard. bIsolated yield from 20 mmol of 1 and 20 h reaction time. cIsolated yield over two steps after reduction of the ketone.