Abstract
Malignant melanoma cells are known to have altered expressions of growth factors as compared to normal melanocytes. Thrombomodulin (TM) is a thrombin receptor on endothelial cells that converts thrombin from a procoagulant to an anticoagulant enzyme. TM expression is downregulated in tumor cells, and this phenomenon correlates with tumor cell invasiveness and a poor prognosis in cancer patients. In this study, we evaluated TM expression in two human melanoma cell lines that are known to have either low (WM35) or high (A375) aggressive phenotypes. Analysis by quantitative real-time PCR (qPCR) showed that the mRNA expression of TM is modestly (WM35) or dramatically (A375) downregulated in melanoma cells, as compared to human primary melanocytes. TM expression levels inversely correlated with in vitro migration properties of tumor cells. In addition, interleukin-8 (IL-8) expression also correlated with the degree of aggressiveness, as indicated by high expression levels of this cytokine in A375 cells. Overexpression of TM in A375 cells by transient transfection reversed their aggressive phenotype and dramatically decreased IL-8 expression by these cells. Taken together, these results suggest that down-regulation of TM plays a crucial role in melanocyte transformation and melanoma progression.
Keywords: thrombomodulin, melanoma, cell migration, interleukin-8, blood coagulation
Introduction
Malignant melanoma is an aggressive tumor that arises from melanocytes and is resistant to most current therapeutic approaches [1]. Metastatic melanoma develops when tumor cells dissociate from the primary lesion, migrate through the surrounding stroma and invade blood and lymphatic vessels to form a tumor at a distant site [2]. Invasion and spread of melanoma are related to alterations in adhesion properties of cells that regulate their migration, tissue organization, and organogenesis [3].
An important link between cancer and coagulation has long been established [4,5]. The clotting initiator protein tissue factor (TF) is expressed in many types of cancer cells. Additionally, research on cultured cells and patient specimens point to a strong correlation between TF expression level and aggressive tumor behavior [6–8]. The TF procoagulant activity is known to be associated with tumor cells suggesting that the coagulation system contributes to pro-tumoral responses, metastasis, modulation of the inflammatory microenvironment and thrombosis [9–12]. By contrast the anticoagulant potential of tumor cells inversely correlates with their aggressive phenotype [13].
Thrombomodulin (TM) functions as receptor for the coagulation protease thrombin and plays critical modulatory roles in inflammation, thrombosis, and carcinogenesis [13–15]. TM is expressed on several human tissues, including endothelial cells, syncytiotrophoblasts of the placenta, platelets, megakaryocytes, leukocytes, mesothelium, keratinocytes, and astrocytes [16,17]. The complex formation of TM with thrombin switches the substrate specificity of protease, thereby inhibiting its cleavage of prothrombotic substrates, which include fibrinogen, factor V, factor VIII, factor XI, factor XIII, and protease-activated receptors (PARs). The TM-thrombin complex promotes the proteolytic activation of the anti-coagulant/antiinflammatory protease zymogen, protein C, and the carboxypeptidase zymogen, thrombin-activatable fibrinolysis inhibitor (TAFI) [14,18,19]. Thus, TM has been considered an important natural protective receptor involved in the maintenance of the fluidity of circulating blood.
In addition to its marked role in regulating the clotting cascade, TM plays an important role in tumor biology, as its increased expression within tumor tissue correlates with better prognosis in colorectal [20], lung [21], prostate [22] and breast cancers [23]. Several studies have postulated that modifying TM expression by tumor cells can alter key aspects of the transformed phenotype, including tumor cell migration and invasion in vitro and in vivo [24–26]. The antiinflammatory effect of TM appears to be mediated, at least in part, by its N-terminus lectin-like domain [27]. However, research has indicated that the anti-proliferative effects of TM on tumor cells also require cytoplasmic and/or transmembrane domains [28].
To understand the protective role of TM in melanocytes, we measured TM expression levels in different melanoma cell lines and in primary cultured melanocytes. We found that TM expression inversely correlates with the aggressive melanoma phenotype, as measured by in vitro migration assays. TM levels were found to inversely correlate with TF procoagulant activity and IL-8 levels. Furthermore, enforced TM expression in A375 cells by transient transfection decreased IL-8 expression and migration properties of this aggressive melanoma cell line. On the basis of these findings, we propose that down-regulation of TM may be associated with melanocyte transformation and melanoma progression.
Materials and methods
Proteins
Human protein C (PC), human activated protein C (APC), catalytically inactive Ser-195 to Ala substitution mutant of protein C and thrombin were prepared as described [29].
Cell culture
Primary epidermal melanocytes and A375 cell line were purchased from ATCC (Manassas, VA). WM35 cell line was purchased from Wistar Institute Collection (Philadelphia, PA). Primary epidermal melanocytes were grown in Dermal Cell Basal Medium (ATCCR PCS-200-030) supplemented with Adult Melanocyte Growth Kit (ATCCR PCS-200-042). The A375 cell line was grown in DMEM (Dulbecco’s Modified Eagle Medium, Life Technologies) supplemented with 10% FBS (Fetal Bovine Serum). The WM35 cell line was grown in 4:1 ratio of MCDB153 (M7403, Sigma-Aldrich) and Leibovitz L-15 (L1518, Sigma-Aldrich) containing 1.68 mM CaCl2 and 5 μg/mL insulin (I9278, Sigma-Aldrich) and 2% of FBS. All cell lines were grown at 37°C in a humidified, 5% CO2 atmosphere in culture flasks.
TM transient expression
The TM cDNA was inserted into HindIII/XbaI cloning sites of the mammalian expression vector pRc/RSV (Invitrogen, San Diego, CA) and transfected to A375 cells (80% of confluence) using the lipofectin reagent (Invitrogen, San Diego, CA). 24 h after transfection, cells were transferred into assay plates. The transfected A375 cell line was designated A375-TM.
Tumor cell tranendothelial migration assay
Migration assays were performed in transwell plates of 6.5 mm diameter, with 8 μm pore size filters (Corning, Lowell, MA). Transformed human umbilical vein endothelial (EA.hy926) cells (1 × 105) (obtained from Dr. C. Edgell from University of North Carolina at Chapel Hill, NC) were grown for 24 h at 37°C to obtain confluent monolayers. The inserts were washed twice with PBS. Melanocytes, WM35, A375 and A375-TM cells (2 × 105) were resuspended in serum free media and added to the upper compartment. FBS (10%) was added as a chemoattractant in the lower chamber. After incubation for 24 h, membranes were washed with PBS. The upper side of the membrane was gently wiped with a cotton swab and fixed with methanol. The membrane was then stained with 0.2% crystal violet (Sigma, St. Louis, MO) in 2% ethanol. Each experiment was repeated in duplicate wells and cell counting was done in four randomly selected microscopic high-power fields. In some cases, EA.hy926 cells were previously exposed to the following proteins: APC (20 nM) or thrombin (2 nM) or PC (80 nM) or PC (80 nM) plus thrombin (2 nM). Cells were treated for 4 h, at 37°C, in a humidified, 5% CO2 atmosphere. EA.hy926 cells were further washed with PBS and A375 cells (2 × 105) were added to the upper compartment.
RNA extraction and real-time PCR
Total RNA was isolated from cultured cells (2.5 × 105) using the Trizol reagent (Invitrogen) following the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed into cDNA using cDNA Archive (Applied Biosystems, Carlsbad, CA, USA) according to the manufacture’s protocol. A 1:10 template dilution of the resulting cDNA was used to quantify the relative content of mRNA by qRT-PCR with Syber Green PCR Master Mix (Applied Biosystems, Foster City, CA) using the PTC 200 real-time PCR detection system. The specific primers for EPCR (5′Forward- TCC GGA GTG GTC ACC TTC A-3′; 5′Reverse- GGA ATT CCC GCA GTT CAT ACC-3′) and GAPDH (5′Forward- ACC CAC TCC TCC ACC TTT GA-3′; 5′Reverse- CTG TTG CTG TAG CCA AAT TCG T-3′) were designed with Oligo Primer Analyses Software (Cascade, CO, USA) and synthesized by IDT (IA, USA). A melt-analysis was run for all products to evaluate the specificity of the amplification. Evaluation of TM, TF and IL-8 expression was performed by qRT-PCR with TaqMan® Universal PCR Master Mix (Applied Biosystems). TM, TF, IL-8 and GAPDH mRNA levels were measured using Pre-developed TaqMan® Gene Expression Assays: Hs00264920_s1 (human Thbd), Hs01076029_m1 (human F3), Hs00174103_m1 (human IL-8) and Hs03929097_g1 (human GAPDH), respectively. The expression level of each gene was normalized to the expression level of the endogenous control (GAPDH) and expressed as the fold change relative to the control group at each time point using the 2−ΔCt method as described [30]. Each sample was measured in triplicate. The mRNA (no reverse transcription) or H2O (no DNA sample) were included in the real-time PCR reaction as negative controls.
Factor X (FX) activation
Activation of FX by factor VIIa (FVIIa) was carried out in 50 mM HEPES, 100 mM NaCl, 5 mM CaCl2, 1 mg/mL BSA, pH 7.5 (HEPES-BSA buffer) in a 48-well plate as follows: FVIIa (1 nM, final concentration) was incubated with cells (5 × 104) at 37 °C in the absence and presence of plasma-derived FX (150 nM, final concentration). Following 30 min incubation, aliquots of 25 μL were removed into a 96-well microplate containing 25 μL of Tris-EDTA buffer (50 mM Tris-HCl, 150 mM NaCl, 20 mM EDTA, 1 mg/mL polyethylene glycol 8000, pH 7.5). After addition of 50 μL of the chromogenic substrate, Spectrozyme FXa, absorbance at 405 nm was recorded, at room temperature, for 5 min using a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA, USA). The total amount of FXa generated in each well was calculated from a standard curve prepared by using calibrated concentrations of FXa.
Flow cytometry
Flow cytometric analyses were performed in order to determine TM expression in melanoma cells. Briefly, 2.5 × 105 cells were washed with phosphate buffered saline (PBS) and further fixed in 0.1% paraformaldehyde. Cells were further stained for 30 min at 4 °C with mouse anti-human TM conjugated with allophycocyanin (Biolegend, USA) in PBS containing 5% FBS. After the incubation period, cells were washed with PBS and analyzed by FACSCalibur flow cytometer (Becton and Dickinson). Ten thousand cells were acquired on the basis of forward and side scatter. Data analyses were performed via Summit 4.3 software.
Statistical analysis
Comparison between groups was made using the Student’s unpaired t-test or One-way ANOVA test. All calculations were performed using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
Results
Analysis of melanoma cell invasiveness
To evaluate invasive properties of different stages of melanoma cells, we established a transmigration assay and monitored the migration of primary melanocytes, WM35 (as a model of in-situ melanoma) and A375 cells (as a model of malignant melanoma) across EA.hy926 endothelial cell monolayers. Elevated cell migration in this model is a hallmark of the metastatic phenotype. As shown in Fig. 1, the ability of A375 melanoma cells and to a lesser extent WM35 cells to migrate toward the chemotactic stimuli was dramatically improved (142 ± 42 and 72 ± 45 migrated cells per field, respectively). In contrast to melanoma cell lines, primary melanocytes exhibited insignificant migratory properties (10 ± 5 migrated cells per field).
Figure 1. In vitro cell migration assay.
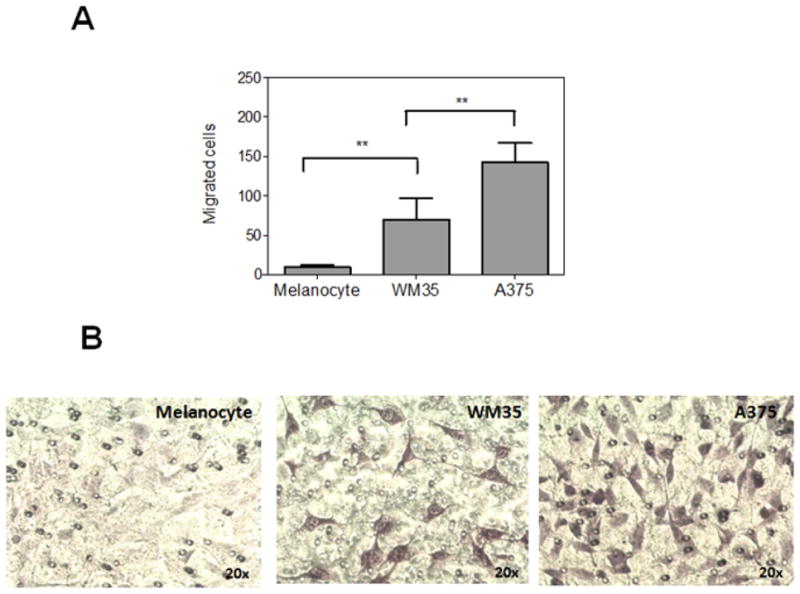
(A) Primary melanocytes and the indicated melanoma cell lines were analyzed for their ability to migrate toward the chemoattractant (conditioned medium containing 10% FBS) across EA.hy926 endothelial cell monolayers as descried in “Material and methods”. Each bar represents the number of migrated cells per well (n=3), counted in 4 fields. **p < 0.01. (B) Representative pictures of each cell line.
Expression of IL-8, TM and EPCR in melanoma cell lines
The migration properties of melanoma cells have been demonstrated to correlate with the production of IL-8. Indeed, in malignant melanoma, IL-8 level has been found to be significantly up-regulated which correlates with an advanced disease stage and overall patient survival [31,32]. In this context, we employed Real-time PCR (qRT-PCR) to analyze mRNA levels of IL-8 in primary melanocytes and melanoma cells. Results presented in Fig. 2A show that melanocytes express a very low level and WM35 melanoma cells express intermediate levels of IL-8. By contrast, A375 cells express high levels of this cytokine. Therefore, IL-8 levels correlate with the degree of cell migration in vitro.
Figure 2. Analysis of IL-8, TM and EPCR expression.
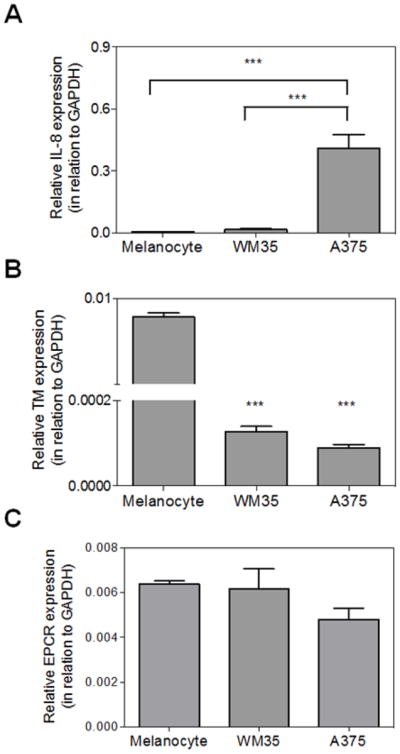
Quantitative analyses of (A) IL-8, (B) TM and (B) EPCR gene expression were performed by qRT-PCR, as described in “Material and methods”. Bars represent mean ± SD of three independent experiments. *** p < 0.001 relative to melanocytes.
Loss of TM expression in tumor cell lines has been associated with increased motility and invasion in vitro [24–26]. Thus, we analyzed expression levels of TM in primary melanocytes and melanoma cells. Results presented in Fig. 2B demonstrate that WM35 and A375 cells express significantly lower levels of TM when compared to primary melanocytes. Flow cytometric analyses of TM antigen demonstrate that WM35 exhibits a 4-fold increased global geometric mean of fluorescence intensity (MFI) in relation to A375 cells (data not shown). In addition to TM, endothelial protein C receptor (EPCR) also contributes to activation of the anticoagulant protein C pathway by thrombin [33]. However, analysis of results presented in Fig. 2C suggests that differences in the EPCR expression levels are not statistically significant between melanocytes and melanoma cell lines.
Tissue factor expression and procoagulant activity of melanoma cell lines
TF is a 47 kDa transmembrane protein that initiates the coagulation cascade and has been demonstrated to be over-expressed in different tumor types [34]. In this context, we employed qRT-PCR to analyze the mRNA levels of TF in melanocytes and melanoma cell lines (Fig. 3A). Surprisingly, melanocytes expressed more TF mRNA than both melanoma cell lines including the most aggressive cell line, A375 (p < 0.05). The TF procoagulant activity level was further evaluated employing a specific enzymatic assay by monitoring activation of exogenously added FX by FVIIa on the surface of these cell lines. Interestingly, results of this functional assay did not show a correlation between the TF mRNA expression level and its cell surface procoagulant activity. Thus, the activation rate of FX by FVIIa was the highest on the surface of A375 cells (Fig. 3B). FX activation was strictly FVIIa-dependent, indicating that assembly of the protease into the extrinsic tenase complex (FVIIa-TF) at the cell surface is responsible for FX activation. No significant FX activation was observed in the absence of FVIIa (Fig. 3B). These results suggest that the more aggressive A375 cells support FX activation more efficiently than both WM35 and melanocytes. The basis for enhanced FX activation by A375 cells is not known. Whether the total cell surface TF level of A375 cells is higher or if TF is differentially in a decrypted form [35] in the aggressive melanoma cells needs further investigation. Taken together, these results demonstrate that, melanoma progression correlates with increased functional cell surface TF activity, suggesting a procoagulant phenotype for metastatic cells.
Figure 3. Analysis of TF expression and procoagulant activity.
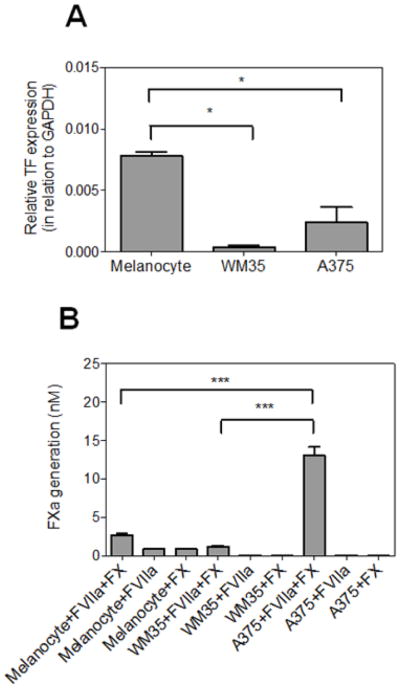
(A) qRT-PCR analysis of TF gene expression. Quantitative gene expression analysis was performed as described in “Material and methods”. Bars represent mean ± SD of three independent experiments. * p < 0.05. (B) Analysis of TF procoagulant activity. Activation of FX by the extrinsic tenase complex assembled on primary melanocytes, WM35 or A375 cell lines was performed as described in “Material and methods”. Bars represent mean ± SD of three independent experiments. *** p < 0.0001.
Enforced TM expression decreases A375 aggressive phenotype
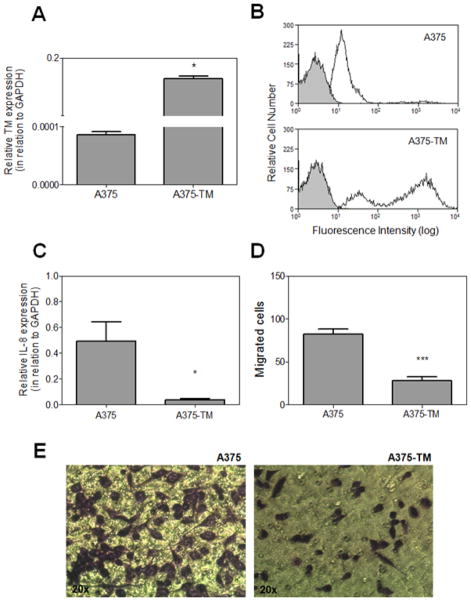
TM expression within tumor tissue correlates with a better prognosis in cancer patients [20,21]. Further support for this hypothesis was provided by transfecting A375 cells with a construct expressing TM. The over-expression of TM was confirmed by qPCR analysis (Fig. 4A). Flow cytometric analyses confirmed the higher level of TM antigen on the surface of A375-TM (Fig. 4B) which presented an approximately 20-fold increase in global geometric mean of fluorescence intensity (MFI) in relation to non-transfected A375 cells. Remarkably, Fig. 4C shows that enforced TM expression by A375 cells dramatically decreases IL-8 expression. In addition, A375-TM showed markedly decreased capacity to migrate across the endothelial cell monolayer (Fig. 4D and Fig. 4E) when compared to non-transfected tumor cells (28 ± 11 and 82 ± 11 migrated cells per field, respectively).
Figure 4. Effect of enforced TM expression on A375 phenotype.
(A) qRT-PCR analyses confirmed the efficient TM transfection in A375-TM cells as compared to non-transfected A375 cells. Bars represent mean ± SD of three independent experiments. *p <0.05 (Student’s t-test). (B) Analysis by flow cytometry of TM expression. Black line represents staining with monoclonal anti-human TM antibody labeled with allophycocyanin. Gray region represents control cells with no staining. Representative histograms of five independent experiments are presented. (C) qRT-PCR analyses of IL-8 expression. Bars represent mean ± SD of five independent experiments. * p < 0.05 (Student’s t-test). (D) A375 migration was determined by transwell assay as described in “Materials and methods”. *** p < 0.0001 (Student’s t-test). Each bar represents the number of migrated cells per well (n=3), counted in 4 fields. (E) Representative pictures of each cell line.
Thrombin enhances and APC inhibits melanoma cell migration
In light of the observation that cell migration and invasive melanoma progression showed a direct correlation with TF procoagulant activity, but an inverse correlation with the expression level of TM, we decided to investigate the migration properties of A375 cells across endothelial monolayer stimulated with either thrombin or activated protein C (APC). It has been demonstrated that these two procoagulant and anticoagulant proteases elicit paradoxical proinflammatory and antiinflammatory responses in endothelial cells, respectively [36]. Both proteases have been demonstrated to exert their intracellular signaling effects through the activation of PAR-1 [36]. Results presented in Fig. 5A show that pre-treatment of endothelial cells with thrombin significantly improves migratory properties of A375 cells. By contrast, APC exhibits a protective activity by significantly inhibiting the migration of melanoma cells across the APC-pretreated endothelial cell monolayer (Fig. 5A). Interestingly, the occupancy of endothelial EPCR by protein C is sufficient to elicit a protective effect since pretreatment of cells with the catalytically inactive Ser-195 to Ala substitution mutant of protein C switches the signaling specificity of thrombin. This is evidenced by thrombin inhibiting migratory properties of melanoma cells across the endothelial cell monolayer (Fig. 5B). These findings support the overall hypothesis that the procoagulant pathway promotes cancer progression but the anticoagulant protein C pathway plays a protective role in the metastatic process [37].
Figure 5. Treatment of endothelial cells with thrombin or activated protein C differentially modulates melanoma cell migration.
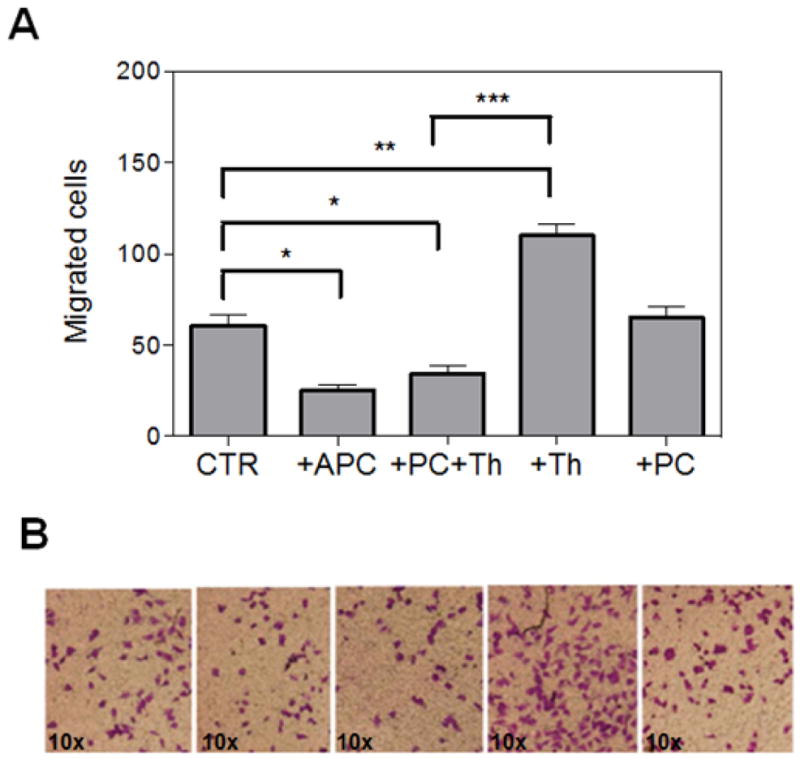
(A) Migration of A375 cells was determined by transwell assay as described in “Material and methods”. EA.hy926 endothelial cells were treated (4 h) with the following proteins: APC (20 nM), thrombin (Th, 2 nM), PC (80 nM), or PC (80 nM) plus thrombin (Th, 2 nM) prior to addition of melanoma cells to the upper compartment. * p < 0,05, ** p < 0.01, *** p < 0.0001. Each bar represents the number of migrated cells per well, counted in 4 fields. (B) Representative pictures of each treatment condition.
Discussion
A correlation between tumor metastasis and blood coagulation has been well established. Thus, an elevated level of TF procoagulant activity has been shown to be associated with efficient metastasis of several cancer cell types including melanoma [7, 8,12]. In this study, we used two melanoma cell lines at different stages of invasiveness (WM35 as a non-metastatic model and A375 as a metastatic model) to determine whether the procoagulant potential of these tumor cells correlates with migration and aggressive phenotype. The procoagulant potential of A375 cells was markedly improved as evidenced by these cells supporting the FVIIa/TF-mediated activation of FX on their surfaces. It was interesting to note that, although the mRNA level of A375 cells was significantly higher than that of the less aggressive WM35 cells, nevertheless, its TF expression level was less than the mRNA level detected in normal melanocytes. The basis for the lack of a correlation between TF mRNA levels and procoagulant phenotypes of these cells was not investigated. However, assuming that high mRNA levels of melanocytes translate to a similar higher cell surface protein expression level, the results may suggest that the majority of cell surface TF on melanocytes but not on A375 cells are in an inert and encrypted state [35]. This hypothesis is consistent with the report that cancer cells lack this regulatory pathway [38]. It is worth noting that other studies have also reported a high TF expression level in melanocytes, but TF levels have not been found to correlate with the metastatic status in tumor cells derived from melanoma patients [39,40].
Several studies suggest that the expression level of the anticoagulant receptor TM inversely correlates with metastasis and tumor progression [21–26]. Remarkably, most human melanomas lack TM expression, a process associated with epigenetic mechanisms [41]. In this study, we observed that the expression level of TM is markedly down-regulated in A375 malignant melanoma when compared to normal human melanocytes. Further support for an important role for the loss of TM in melanoma progression was provided by the observation that the transient transfection of A375 cells with a construct coding for human TM inhibited the capacity of these cells to effectively migrate across the endothelial monolayer, reflecting a decreased metastatic phenotype for cells expressing elevated levels of TM. These results are consistent with the study performed by Kao and colleagues which demonstrated that the expression levels of TM inversely correlate with tumor cell migration in vitro in a carcinoma model [26]. We observed that enforced expression of TM strongly interfered with IL-8 expression by A375 cells. Expression of IL-8 by melanoma cells has been shown to correlate with in vitro migratory properties and metastatic potential in vivo [31,32,42–44]. In addition, increased IL-8 levels have been observed in serum of patients with metastatic melanoma, inversely correlating with survival [45–46]. Taken together, loss of TM during melanoma progression may trigger a transcriptional program involving distinct genes associated with the metastatic phenotype.
Thrombotic events have been demonstrated to promote metastasis in several animal models [47,48]. Thus, TM can exert its anti-metastatic effect by inhibiting thrombin generation under in vivo settings [24,25]. However, in the absence of exogenously added thrombin, the mechanism by which the enforced expression of TM in A375 cells inhibits cell motility and migratory properties of melanoma cells is not known. Nevertheless, TM has been shown to play an important protective role in modulating inflammatory responses [14,15,18–20]. Thus, it has been found that soluble TM can inhibit migratory properties of murine B16F10 melanoma cells by a thrombin-independent mechanism. The inhibitory effect of TM in inhibiting B16F10 cell migration has been demonstrated to require the lectin-like domain of the receptor as evidenced by lack of an inhibitory effect for a recombinant TM fragment lacking this domain [25]. This has raised the possibility that the interaction of the lectin-like domain with a cell surface molecule/receptor modulates the expression of proinflammatory cell adhesion molecules involved in the invasion and cell migration process [27]. In support of an antiinflammatory signaling function for TM, it has been demonstrated that the lectin-like domain suppresses the expression of cell adhesion molecules through modulation of the NF-kB and MAP kinase pathways in vitro and in vivo settings [26]. Thus, the enforced expression of TM on A375 melanomas is likely down-regulating the expression of cell adhesion molecules/integrins involved in promoting the adhesion-dependent migration of these cells across the endothelial monolayer in our assay system.
The other interesting finding of this study is the observation that the pretreatment of endothelial cells with APC also potently inhibits migratory properties of A375 cells. It is known that APC can enhance the vascular barrier function and inhibit expression of cell adhesion molecules through the inhibition of the NF-kB pathway [49]. Noting that the vascular hyperpermeability and expression of adhesion molecules on tumor cells are prerequisite steps in metastasis, the results suggest that, in addition to its anticoagulant role, APC plays a critical protective role in inhibiting melanoma progression through its antiinflammatory function. The antiinflammatory function of APC is mediated through the protease binding to EPCR and activating PAR-1 [36], thereby promoting the cellular barrier function and inhibiting the NF-kB-dependent up-regulation of cell adhesion molecules and cytokines (i.e., IL-8) that are involved in cell migration and extravasation [31]. By contrast to APC, the activation of PAR-1 by thrombin elicits opposite proinflammatory responses and thus promotes metastasis by stimulating the expression of adhesion molecules and matrix metalloproteases involved in the degradation of a variety of extracellular matrix (ECM) proteins [50,51]. However, we have demonstrated that PAR-1, EPCR and TM are all colocalized within membrane lipid-rafts and that the occupancy of either EPCR by protein C or TM by thrombin alters the signaling specificity of PAR-1, thus the activation of PAR-1 by both APC and thrombin elicits antiinflammatory responses as long as one or both of related receptors are occupied by their natural ligands [52]. In agreement with this hypothesis, the thrombin treatment of endothelial cells promoted the migratory potential of A375 cells, however, pretreatment of endothelial cells with the catalytically inactive protein C zymogen, switched the signaling specificity of thrombin to a protective response, thereby inhibiting the migration of melanoma cells. Thus, the anticoagulant receptors can play key physiological roles in inhibiting the migration of circulating tumor cells, which may have escaped immune surveillance, across the vascular endothelium, thereby inhibiting their metastatic potential. Taken together, these results suggest metastasis and melanoma progression have positive correlation with procoagulant/proinflammatory and negative correlation with anticoagulant/antiinflammatory phenotypes.
Acknowledgments
We thank Audrey Rezaie for proofreading the manuscript. Vitor Hugo Almeida and Araci Rondon (UFRJ) for technical assistance. This research was supported by Brazilian National Council for Scientific and Technological Development (CNPq), The State of Rio de Janeiro Research Foundation (FAPERJ), Brazilian Cancer Foundation (Fundação do Cancer) and grants awarded by the National Heart, Lung, and Blood Institute of the National Institute of Health HL 101917 and HL 62565 to ARR.
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interest.
References
- 1.Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA, et al. Melanoma: molecular pathogenesis and emerging target therapies. Int J Oncol. 2009;34:1481–1489. doi: 10.3892/ijo_00000277. [DOI] [PubMed] [Google Scholar]
- 2.Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- 4.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:215–224. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 5.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumor progression. Biosci Rep. 2013 doi: 10.1042/BSR20130057. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro FS, Simão TA, Amoêdo ND, Andreollo NA, Lopes LR, Acatauassu R, Rumjanek FD, Albano RM, Pinto LF, Monteiro RQ. Evidence for increased expression of tissue factor and protease-activated receptor-1 in human esophageal cancer. Oncol Rep. 2009;21:1599–1604. doi: 10.3892/or_00000393. [DOI] [PubMed] [Google Scholar]
- 7.Maciel EO, Carvalhal GF, da Silva VD, Batista EL, Garicochea B. Increased tissue factor expression and poor nephroblastoma prognosis. J Urol. 2009;182:1594–1599. doi: 10.1016/j.juro.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Mueller BM, Reisfeld RA, Edgington TS, Ruf W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc Natl Acad Sci (USA) 1992;89:11832–11836. doi: 10.1073/pnas.89.24.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo JS, Degen JL. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb Res. 2007;120 (Suppl 2):S22–28. doi: 10.1016/S0049-3848(07)70127-5. [DOI] [PubMed] [Google Scholar]
- 10.Kirszberg C, Lima LG, Da Silva de Oliveira A, Pickering W, Gray E, Barrowcliffe TW, Rumjanek VM, Monteiro RQ. Simultaneous tissue factor expression and phosphatidylserine exposure account for the highly procoagulant pattern of melanoma cell lines. Melanoma Res. 2009;19:301–308. doi: 10.1097/CMR.0b013e32832e40fe. [DOI] [PubMed] [Google Scholar]
- 11.Lima LG, Oliveira AS, Campos LC, Bonamino M, Chammas R, Werneck C, Vicente CP, Barcinski MA, Petersen LC, Monteiro RQ. Malignant transformation in melanocytes is associated with increased production of procoagulantmicrovesicles. Thromb Haemost. 2011;106:712–723. doi: 10.1160/TH11-03-0143. [DOI] [PubMed] [Google Scholar]
- 12.da de Oliveira AS, Lima LG, Mariano-Oliveira A, Machado DE, Nasciutti LE, Andersen JF, Petersen LC, Francischetti IM, Monteiro RQ. Inhibition of tissue factor by ixolaris reduces primary tumor growth and experimental metastasis in a murine model of melanoma. Thromb Res. 2012;130:163–170. doi: 10.1016/j.thromres.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanly AM, Winter DC. The role of thrombomodulin in malignancy. Semin Thromb Hemost. 2007;33:673–679. doi: 10.1055/s-2007-991969. [DOI] [PubMed] [Google Scholar]
- 14.Esmon CT, Fukudome K, Mather T, Bode W, Regan LW, Stearns-Kurosawa DJ, et al. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–259. [PubMed] [Google Scholar]
- 15.Koutsi A, Papapanagiotou A, Papavassiliou AG. Thrombomodulin: from haemostasis to inflammation and tumourigenesis. Int J Biochem Cell Biol. 2008;40:1669–1673. doi: 10.1016/j.biocel.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Fink LM, Eidt JF, Johnson K, Cook JM, Cook CD, Morser J, et al. Thrombomodulin activity and localization. Int J Dev Biol. 1993;37:221–226. [PubMed] [Google Scholar]
- 17.Raife TJ, Lager DJ, Madison KC, Piette WW, Howard EJ, Sturm MT, Chen Y, Lentz SR. Thrombomodulin expression by human keratinocytes.. Induction of cofactor activity during epidermal differentiation. J Clin Invest. 1994;93:1846–1851. doi: 10.1172/JCI117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiler H, Isermann BH. Thrombomodulin. J Thromb Haemost. 2003;1:1515–1524. doi: 10.1046/j.1538-7836.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiler H. Mouse models of thrombosis: thrombomodulin. Thromb Haemost. 2004;92:467–477. doi: 10.1160/TH04-05-0307. [DOI] [PubMed] [Google Scholar]
- 20.Hanly AM, Redmond M, Winter DC, Brophy S, Deasy JM, DJ, Bouchier-Hayes DJ, Kay EW. Thrombomodulin expression in colorectal carcinoma is protective and correlates with survival. Br J Cancer. 2006;94:1320–1325. doi: 10.1038/sj.bjc.6603098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu PL, Tsai JR, Chiu CC, Hwang JJ, Chou SH, Wang CK, et al. Decreased expression of thrombomodulin is correlated with tumor cell invasiveness and poor prognosis in nonsmall cell lung cancer. Mol Carcinog. 2010;49:874–881. doi: 10.1002/mc.20663. [DOI] [PubMed] [Google Scholar]
- 22.Menschikowski M, Hagelgans A, Tiebel O, Vogel M, Eisenhofer G, Siegert G. Regulation of thrombomodulin expression in prostate cancer cells. Cancer Lett. 2012;322:177–184. doi: 10.1016/j.canlet.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Niimi S, Harashima M, Takayama K, Hara M, Hyuga M, Seki T, Ariga T, Kawanishi T, Hayakawa T. Thrombomodulin enhances the invasive activity of mouse mammary tumor cells. J Biochem. 2005;137:579–586. doi: 10.1093/jb/mvi070. [DOI] [PubMed] [Google Scholar]
- 24.Huang MT, Wei PL, Liu JJ, Liu DZ, Huey-Chun H, An J, Wu CC, Wu CH, Ho YS, Yang YY, Chang YJ. Knockdown of thrombomodulin enhances HCC cell migration through increase of ZEB1 and decrease of E-cadherin gene expression. Ann Surg Oncol. 2010;17:3379–3385. doi: 10.1245/s10434-010-1163-4. [DOI] [PubMed] [Google Scholar]
- 25.Hosaka Y, Higuchi T, Tsumagari M, Ishii H. Inhibition of invasion and experimental metastasis of murine melanoma cells by human soluble thrombomodulin. Cancer Lett. 2000;161:231–240. doi: 10.1016/s0304-3835(00)00617-0. [DOI] [PubMed] [Google Scholar]
- 26.Kao YC, Wu LW, Shi CS, Chu CH, Huang CW, Kuo CP, Sheu HM, Shi GY, Wu HL. Downregulation of thrombomodulin, a novel target of Snail, induces tumorigenesis through epithelial-mesenchymal transition. Mol Cell Biol. 2010;30:4767–4785. doi: 10.1128/MCB.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med. 2002;196:565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Weiler-Guettler H, Chen J, Wilhelm O, Deng Y, Qiu F, Nakagawa K, Klevesath M, Wilhelm S, Böhrer H, Nakagawa M, Graeff H, Martin E, Stern DM, Rosenberg RD, Ziegler R, Nawroth PP. Thrombomodulin modulates growth of tumor cells independent of its anticoagulant activity. J Clin Invest. 1998;101:1301–1309. doi: 10.1172/JCI925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Manithody C, Rezaie AR. Contribution of basic residues of the 70–80-loop to heparin binding and anticoagulant function of activated protein C. Biochemistry. 2002;41:6149–6157. doi: 10.1021/bi015899r. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 32.Bar-Eli M. Role of interleukin-8 in tumor growth and metastasis of human melanoma. Pathobiology. 1999;67:12–18. doi: 10.1159/000028045. [DOI] [PubMed] [Google Scholar]
- 33.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci (USA) 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rak J, Milsom C, Magnus N, Yu J. Tissue factor in tumour progression. Best Pract Res Clin Haematol. 2009;22:71–83. doi: 10.1016/j.beha.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Chen VM, Hogg PJ. Encryption and decryption of tissue factor. J Thromb Haemost. 2013;11 (Suppl 1):277–284. doi: 10.1111/jth.12228. [DOI] [PubMed] [Google Scholar]
- 36.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 37.Spek CA, Arruda VR. The protein C pathway in cancer metastasis. Thromb Res. 2012;129 (Suppl 1):S80–84. doi: 10.1016/S0049-3848(12)70022-1. [DOI] [PubMed] [Google Scholar]
- 38.Schaffner F, Ruf W. Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler ThrombVasc Biol. 2009;29:1999–2004. doi: 10.1161/ATVBAHA.108.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kageshita T, Funasaka Y, Ichihashi M, Wakamatsu K, Ito S, Ono T. Tissue factor expression and serum level in patients with melanoma does not correlate with disease progression. Pigment Cell Res. 2001;14:195–200. doi: 10.1034/j.1600-0749.2001.140309.x. [DOI] [PubMed] [Google Scholar]
- 40.Kageshita T, Funasaka Y, Ichihashi M, Ishihara T, Tokuo H, Ono T. Differential expression of tissue factor and tissue factor pathway inhibitor in metastatic melanoma lesions. Pigment Cell Res. 2002;15:212–216. doi: 10.1034/j.1600-0749.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 41.Furuta J, Kaneda A, Umebayashi Y, Otsuka F, Sugimura T, Ushijima T. Silencing of the thrombomodulin gene in human malignant melanoma. Melanoma Res. 2005;15:15–20. doi: 10.1097/00008390-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- 43.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 44.Dong C, Slattery MJ, Liang S, Peng HH. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech. 2005;2:145–159. [PMC free article] [PubMed] [Google Scholar]
- 45.Scheibenbogen C, Möhler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5:179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 47.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–362. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Horowitz NA, Blevins EA, Miller WM, Perry AR, Talmage KE, Mullins ES, Flick MJ, Queiroz KC, Shi K, Spek CA, Conway EM, Monia BP, Weiler H, Degen JL, Palumbo JS. Thrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domain. Blood. 2011;118:2889–2895. doi: 10.1182/blood-2011-03-341222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joyce DE, Gelbert L, Ciaccia A, De Hoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 50.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 51.Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 52.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]