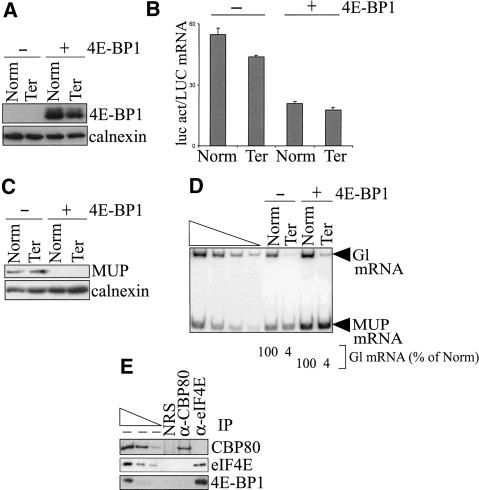
Figure 1.
4E-BP1 inhibits the production of luc activity without abrogating NMD. COS cells were transiently transfected with pmCMV-Gl (Norm or Ter), phCMV-MUP, pGL2, and either pACTAG2 empty vector (-) or expressing 4E-BP1 (+). (A) 4E-BP1 is detected in cells transfected with a 4E-BP1 expression vector (+) but not in cells transfected with empty vector (-) as determined by Western blotting, where the level of endogenous calnexin controlled for variations in the amount of cellular protein analyzed. (B) Luc activity (act), which was normalized to the level of LUC mRNA, is inhibited in cells expressing 4E-BP1. (C) Production of the secreted protein MUP, which was measured relative to the level of calnexin, is inhibited in cells expressing 4E-BP1. (D) The level of nonsense-containing Gl mRNA (Ter) is the same percentage of the level of nonsense-free Gl (Norm) in cells regardless of 4E-BP1 expression. For each lane, the level of Gl mRNA was normalized to the level of MUP mRNA, and the normalized level of Norm was defined as 100. Serial dilutions of RNA in the left four lanes demonstrate that the RT-PCR analysis is semiquantitative. (E) α-eIF4E antibody but not α-CBP80 antibody, or, as a control for nonspecific IP, normal rabbit serum (NRS), immunopurifies 4E-BP1. Serial dilutions of protein in the left three lanes demonstrate that the Western blot analyses of immunopurified proteins using antibodies against CBP80, eIF4E, or 4E-BP1 are semiquantitative. All results are representative of at least two independently performed experiments.