Figure 1.
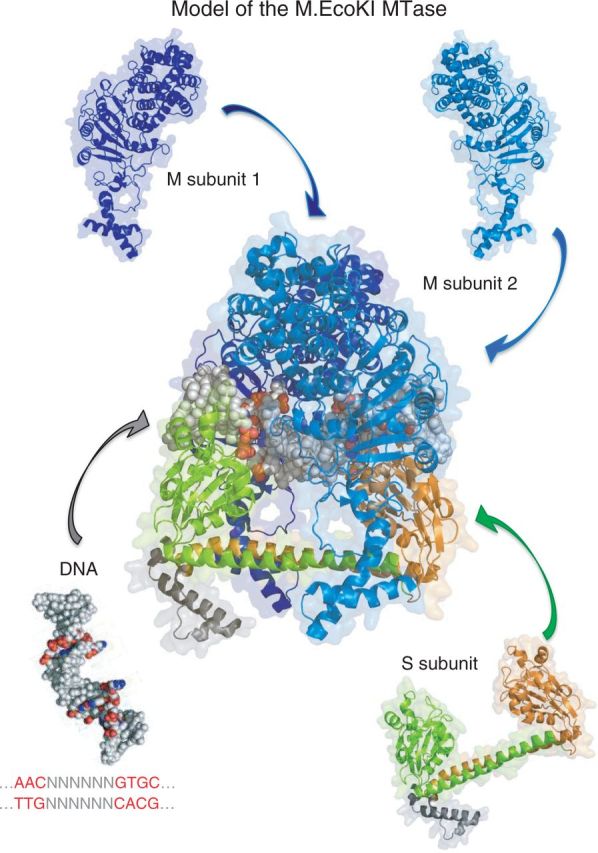
Model of the M.EcoKI MTase (pdb file 2Y7H). The S subunit is composed of two TRDs in inverted orientations. Each TRD comprises a globular DNA-binding domain and an alpha helical dimerization domain. The N-TRD (green) in this protein is specific for the sequence AAC (the 5′-half-sequence), and the C-terminal domain (orange) is specific for GCAC (the 3′ half-sequence). Zipper-like association of the helices separates the globular domains by a fixed distance and reverses the orientation of the C-TRD, resulting in the composite recognition sequence that is bipartite: AAC N6 GTGC. Each TRD also associates with one M subunit (identical, but shown here in different shades of blue for clarity) to form an M2S trimer. Neither S nor M subunits bind to DNA alone, but the trimer binds specifically at the recognition sequence and catalyzes methylation of one adenine in each half-sequence. Because the TRDs are inverted, the two M subunits have opposite orientations. Consequently, both strands of the recognition sequence become methylated, the ‘top’ strand of the 5′ half-sequence (Am6AC) and the ‘bottom’ strand of the 3′ half-sequence (GCm6AC). This enables the DNA of the host cell to be distinguished from infecting DNA during DNA replication.
