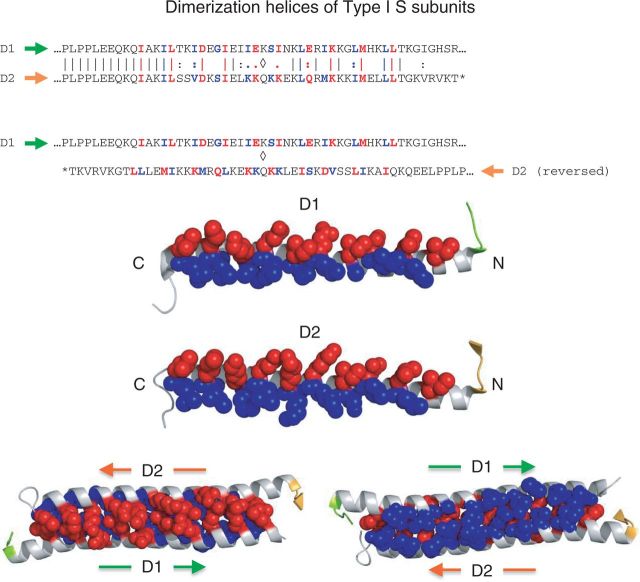
Figure 5.
Dimerization helices of Type I S subunits. Upper diagram: aa sequence alignment of the dimerization helices of S-MjaXI (pdb:1YF2; D1 and D2 in Figure 3) show that they are similar but not identical (top). The proline-rich motif that precedes each helix (IPLPP) is a hallmark of Type I S subunits and likely plays a structural role establishing the correct architectural relationship between the S and D domains. The helices interact in opposite orientations to form an antiparallel coiled-coil (bottom). Adjacent pairs of aa (red and blue) form the dimerization interface and occur with the 4-3-4-3 … spacing characteristic of leucine zippers. Middle diagram: Exposed dimerization surfaces of two helices. Side chains of red aa point ‘up’ toward the bound DNA, and those of blue aa point ‘down’. To form the coiled coil, D1 must be rotated 180 degrees around the vertical axis and docked against the surface of D2 shown. Lower diagram: Within the coiled coil, red aa from one helix interdigitate with red aa from the other helix, forming the ‘upper’ surface of the coiled coil, the surface closest to the bound DNA (left). And blue aa from one helix inter-digitate with blue aa from the other helix to form the ‘lower’ surface on the other side (red). Within the coiled-coiled, red side chains from one helix stack on blue side chains from the other helix in an alternating pattern. At this interface, the helices have complimentary topologies such that a ridge or bump in one is accommodated by a valley or depression in the other, resulting in a close hydrophobic fit.