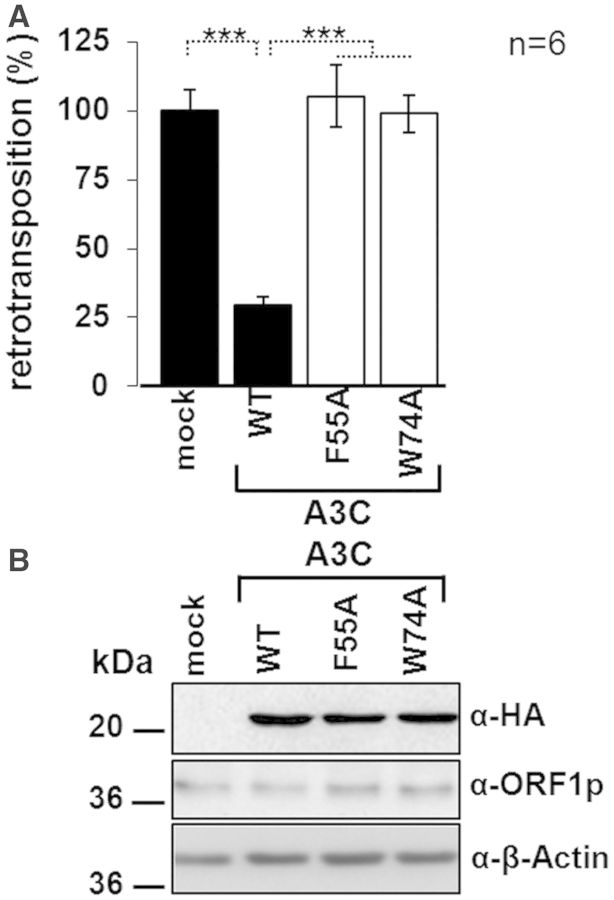
Figure 3.
A3C dimerization is required for L1 restriction by A3C. (A) L1 retrotransposition reporter assays in the presence of A3C dimerization mutant proteins F55A and W74A. The L1 reporter plasmid pJM101/L1RP was co-transfected with expression plasmids coding for A3C-WT or its mutants F55A and W74A. G418R selection for retrotransposition events followed 1 day after transfection. L1 retrotransposition frequencies were determined by counting G418R HeLa colonies. Each co-transfection experiment, and subsequent retrotransposition reporter assay, was carried out twice in triplicates. Relative retrotransposition frequencies are indicated as bar diagram. The number of retrotransposition events obtained after co-transfection of pJM101/L1RP with the empty expression plasmid (mock) was set as 100%. Each bar depicts the arithmetic mean ± SD of the relative retrotransposition frequencies obtained from six individual co-transfection experiments (n = 6). Absolute retrotransposition frequencies and P-values (*P < 0.05, **P < 0.01, ***P < 0.001) are listed in Supplementary Table S1. (B) Immunoblot analysis of A3C and L1 ORF1p expression in HeLa cells after co-transfection of the L1 reporter plasmid with the respective A3C-WT and mutant A3C expression construct (F55A, W47A). Whole-cell lysates were prepared 2 days after co-transfection and subjected to immunoblot analysis using antibodies against L1 ORF1p (α-ORF1p) or HA-tag (α-HA). An amount of 20 µg of whole-cell extract was loaded per lane. β-actin protein levels were analyzed as loading controls.