Figure 4.
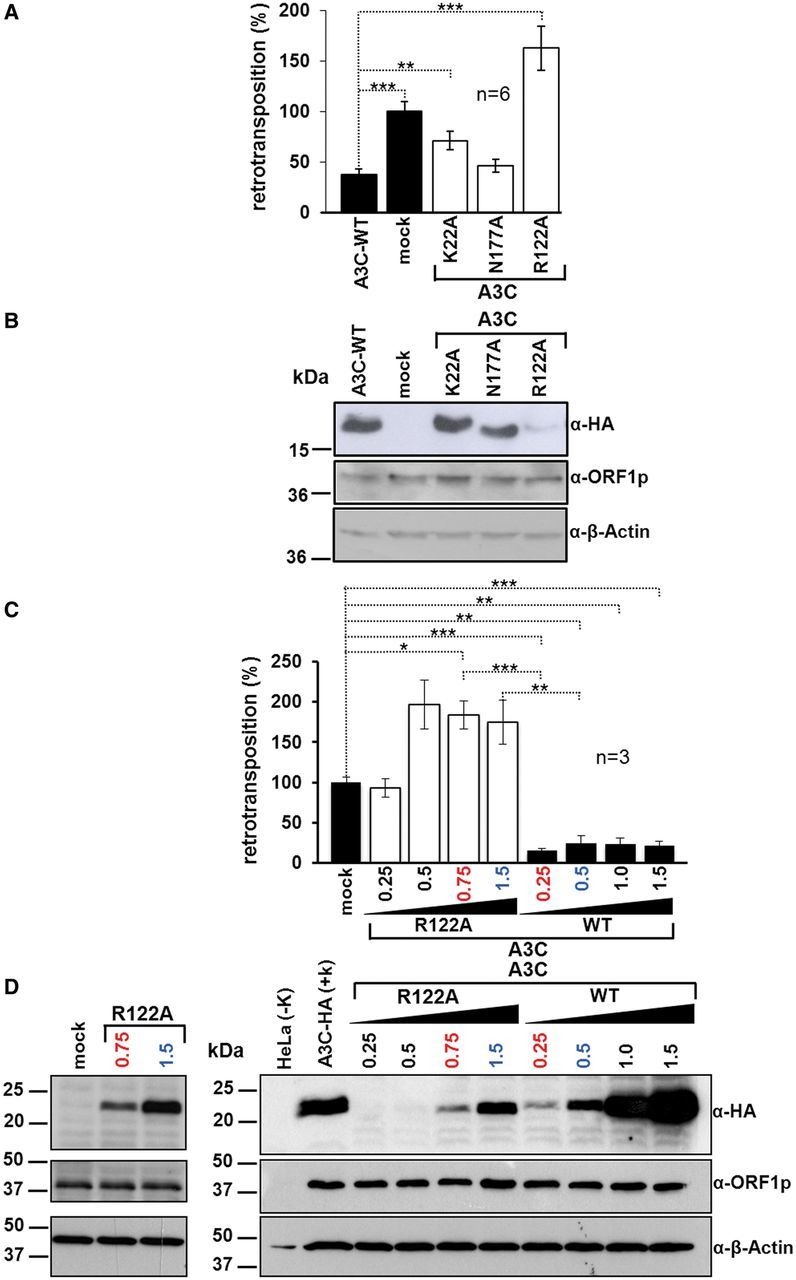
Effects of mutations in the putative RNA-binding pocket of A3C on L1 restriction. (A) Relative L1 retrotransposition frequencies in the presence of ectopically expressed A3C-WT and the mutant proteins K22A, N177A and R122A. HeLa cells were co-transfected with 0.5 µg of both L1 reporter pJM101/L1RP and WT or mutant A3C expression plasmids. After 11 days of G418 selection, the number of G418R colonies was determined. The number of retrotransposition events in the presence of the empty A3C expression plasmid (mock) was set as 100%. Each co-transfection experiment, and subsequent retrotransposition reporter assay, was carried out twice in triplicates. Each bar depicts the arithmetic mean ± SD of the relative retrotransposition frequencies obtained from six individual co-transfection experiments (n = 6). Absolute retrotransposition frequencies and P-values (*P < 0.05, **P < 0.01, ***P < 0.001) are listed in Supplementary Table S1. (B) Immunoblot analysis of the expression of A3C-WT and the mutants K22A, N177A and R122A after co-transfection with the L1 reporter pJM101/L1RP. Expression of the L1 reporter and A3C proteins was detected using antibodies against L1 ORF1p (α-ORF1p) or HA-tag (α-HA), respectively. An amount of 20 µg of whole-cell extracts was loaded per lane. β-actin protein levels were analyzed as loading controls. (C) Titration of A3C-WT and A3C-mutant R122A protein levels against L1 retrotransposition. HeLa cells were co-transfected with 0.5 µg of pJM101/L1RP and variable amounts of A3C-WT (0.25, 0.5, 1 or 1.5 µg) of A3C-R122A mutant-encoding plasmid DNA (0.25, 0.5, 0.75 or 1.5 µg). The total amount of co-transfected plasmid DNA was maintained at 2 µg by adding empty parental A3C expression vector pcDNA3.1/Zeo(+). Following 11 days of G418 selection, the number of G418R colonies was determined. The number of retrotransposition events in the presence of the empty expression plasmid (mock) was set as 100%. Data represent arithmetic means ± SD of three independent experiments. Absolute retrotransposition frequencies and P-values are listed in Supplementary Table S1. (D) Immunoblot analysis of A3C-WT and R122A protein expression during titration. Cell lysates were isolated 3 days after co-transfection of pJM101/L1RP and 0.25–1.5 µg of A3C-WT or the R122A mutant expression plasmid. Expression of the L1 reporter and A3C proteins was detected using antibodies against L1 ORF1p (α-ORF1p) or HA-tag (α-HA), respectively. An amount of 20 µg of whole-cell extracts was loaded per lane. β-actin protein levels were analyzed as loading controls. Comparable amounts of A3C-WT and R122A mutant proteins were observed after transfection of 0.25 µg of A3C-WT and 0.75 µg of R122A expression construct (numbers in red) or after transfection of 0.5 µg of A3C-WT and 1.5 µg of R122A expression construct (numbers in blue), respectively.
