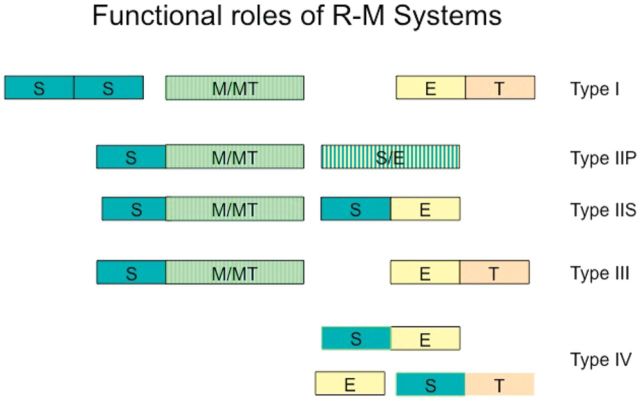
Figure 1.
Schematic representation of the functional roles filled in different ways in R-M systems. The functions served include the following: S, DNA sequence specificity; MT, methyltransferase catalytic activity; M, SAM binding; E, endonuclease; T, translocation. Boxes outline functions that are filled by distinct protein domains. Different colours indicate different functions, while different boxes represent distinct domains. Domains in a single polypeptide are abutted, and those in separate polypeptides spaced apart. The order of domains in a polypeptide may vary—e.g. not all Type IIS enzymes have the cleavage domain at the C-terminus. In some cases, functions are integrated with each other, e.g. S and E functions of Type IIP (striped box); in other cases, separate domains carry them out, e.g. Type IIS. See Table 2 for the complexity and diversity of Type II subtypes. The large families of Type I and Type II systems are currently subdivided in 5 and 11 different groups, respectively. The Type I families are distinguished by homology; the Type II groups are distinguished by catalytic properties rather than sequence homology. Type IV enzymes were initially identified as hydroxymethylation-dependent restriction enzymes and currently comprise a highly diverse family. Two examples are shown with and without a translocation domain.