Abstract
Background
American College of Surgeons Oncology Group (ACOSOG) Z0011 demonstrated that eligible breast cancer patients with positive sentinel lymph nodes (SLN) could be spared an axillary lymph node dissection (ALND) without sacrificing survival or local control. Although heralded as a “practice-changing trial”, some argue that the stringent inclusion criteria limit the trial’s clinical significance. The objective was to assess the potential impact of ACOSOG Z0011 on axillary surgical management of Medicare patients and examine current practice patterns.
Methods
Medicare beneficiaries aged ≥66 with non-metastatic invasive breast cancer diagnosed from 2001–2007 were identified from the Surveillance, Epidemiology and End Results-Medicare database (n=59,431). Eligibility for ACOSOG Z0011 was determined: SLN mapping, tumor <5 cm, no neoadjuvant treatment, breast conservation; number of positive nodes was determined. Actual surgical axillary management for eligible patients was assessed.
Results
12% (6,942/59,431) underwent SLN mapping and were node positive. Overall, 2,637 patients (4.4% (2,637/59,431) of the total cohort but 38% (2,637/6,942) of patients with SLN mapping and positive nodes) met inclusion criteria for ACOSOG Z0011, had 1 or 2 positive lymph nodes, and could have been spared an ALND. Of these 2,637 patients, 46% received a completion ALND and 54% received only SLN biopsy.
Conclusions
Widespread implementation of ACOSOG Z0011 trial results could potentially spare 38% of older breast cancer patients who undergo SLN mapping with positive lymph nodes an ALND. However, 54% of these patients are already managed with SLN biopsy alone, lessening the impact of this trial on clinical practice in older breast cancer patients.
Introduction
Sentinel lymph node (SLN) biopsy is currently the standard of care for staging the clinically negative axilla in breast cancer patients, with axillary lymph node dissection (ALND) reserved for patients with clinical axillary metastases or metastases found on SLN biopsy.1–3 However, this treatment paradigm has changed based on the results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 randomized trial initially reported in 2010.4,5 This trial included patients with clinically node negative invasive breast cancer treated with lumpectomy and whole breast radiation. Patients with 1 or 2 positive SLNs were randomized to undergo completion ALND or observation. In this select group of patients, no significant difference in survival4 or locoregional recurrence5 was observed between the patients undergoing SLN biopsy alone and those who received a completion ALND.
The ACOSOG Z0011 study has been heralded by many as a “practice changing trial,”6–8 altering the treatment paradigm for axillary metastases and sparing eligible women the morbidity of a completion ALND without sacrificing survival or local control. However, others have argued that the stringent inclusion criteria associated with the trial limits the true clinical significance of these findings, as a relatively small proportion of patients will be eligible.9 Given this, we sought to evaluate the potential impact of the ACOSOG Z0011 trial on the axillary surgical management of older breast cancer patients by determining the proportion of Medicare breast cancer patients eligible for ACOSOG Z0011. To further evaluate the potential impact, a secondary objective was to examine current practice patterns with regards to axillary surgical management in this older population.
Methods
This study was approved by the University of Wisconsin Institutional Review Board and granted a waiver of consent.
Data source
The linked Surveillance, Epidemiology and End Results (SEER)-Medicare database was used to identify patients diagnosed with breast cancer from 2001 to2007. The SEER cancer registries include information on patient demographics, tumor characteristics, first course of treatment, and survival for persons newly diagnosed with cancer. For individuals who are eligible for Medicare services, the SEER-Medicare database includes claims for covered health care services, including hospital, physician, outpatient, home health, and hospice bills. The SEER-Medicare dataset has successfully linked 93% of individuals over age 65 at diagnosis to their Medicare record.10,11 In 2000, SEER regions included approximately 26% of the US population. 12 The SEER-Medicare data is an established resource for studying cancer practice patterns in older patients.13
Patient selection
All female, Medicare-enrolled patients aged 66 years and older diagnosed with non-metastatic invasive breast cancer within a SEER region between 2001 and 2007 were eligible. SEER anatomic site (C50.0–50.6, 50.8–50.9), and histology (8000–8005, 8010–8015, 8020–8022, 8030–8035, 8041, 8043, 8050–8052, 8140–8141, 8143, 8190, 8200, 8201, 8211, 8230–8231, 8251, 8255, 8260–8261, 8310, 8314,−8315, 8320, 8323, 8401, 8440, 8480, 8481, 8490, 8500–8504, 8507–8508, 8510, 8512–8514, 8520–8525, 8530, 8540–8541, 8543, 8550, 8551, 8560, 8562, 8570–8575, 8980–8982, 9020) codes were used to identify breast cancer patients. Patients were included in the study if they underwent definitive breast surgery. Continuous enrollment in Medicare Part A and B was required for 1-year preceding diagnosis to establish comorbidities through a minimum of 2-years after diagnosis, death or December 31, 2009 (whichever came first) to assess treatment received. Patients were excluded if they were enrolled in a Health Maintenance Organization (HMO) during the same time period. Patients were also excluded if they were diagnosed with another malignancy five years before or after the date of breast cancer diagnosis, or if their first diagnosis of breast cancer was made after death (i.e., on autopsy or death certificate). The final sample size was 59,431 patients.
Patient-related variables
Basic demographics were obtained from SEER data. The Deyo implementation14 of the Charlson Comorbidity Index15 was used to assess patient comorbidities. The 6th edition American Joint Committee on Cancer (AJCC) staging16 was used to assign stage based on SEER tumor size and number of nodes positive. Estrogen and progesterone receptor (ER/PR) status, and tumor grade were also assessed using SEER.
Treatment-related variables
Receipt of chemotherapy, radiation, and surgical therapy were defined using ICD-9 and CPT codes (Table 1). The most definitive surgery (i.e. mastectomy if it followed lumpectomy) was used to assign surgical management of the breast. We determined definitive axillary surgery based on an algorithm using CPT codes and number of lymph nodes examined (Table 1).
Table 1.
Codes Used to Determine Treatment Received
| Treatment Modality | Definition | Codes Used |
|---|---|---|
| Radiation Therapy | Within 45 days prior or 364 days after breast cancer diagnosis | CPT: 77401–77404, 77406–77409, 77411–77414, 77416, 77418, 77421–77423, 77427, 77431, 77432, 77470, 77499, 77520, 77522, 77523, 77525, 77750, 77761–77763, 77776–77778, 77781–77784, 77789, 77790, 77799 |
| ICD-9: 92.2–92.29, V58.0, G0256, G0261 | ||
| Revenue center: 0330, 0333 | ||
| Chemotherapy | Within 45 days prior or 364 days after breast cancer diagnosis* | CPT: 96400–96402, 96405, 96406, 96408–96417, 96420, 96422, 96423, 96425, 96440, 96445, 96450, 96520–96523, 96530, 96542, 96545, 96549 |
| Health Care Common Procedural Coding System: J0640, J8510, J8520, J8521, J8530, J8560, J8562, J8565, J8597, J8600, J8610, J8700, J8705, J8999–J9001, J9010, J9015, J9017, J9020, J9025, J9027, J9031, J9033, J9035, J9040, J9041, J9043, J9045, J9050, J9055, J9060, J9062, J9065, J9070, J9080, J9090-J9098, J9100, J9110, J9120, J9130, J9140, J9150, J9151, J9155, J9160, J9165, J9170, J9171, J9178–J9179, J9181, J9182, J9185, J9190, J9200–J9202, J9206–J9208, J9211–J9219, J9225, J9226, J9228, J9230, J9245, J9250, J9260-J9268, J9270, J9280, J9290, J9291, J9293, J9300, J9302, J9303, J9305, J9307, J9310, J9315, J9320, J9328, J9330, J9340, J9350, J9351, J9355, J9357, J9360, J9370, J9375, J9380, J9390, J9395, J9600, J9999, Q0083–Q0085 | ||
| Revenue Center codes: 0331, 0332, 0335 | ||
| ICD-9: 99.25, V58.1, V66.2, V67.2, E9931, S9329-S9331, C8953–C8955, G0355–G0363, G9021–G9032 | ||
| Breast Surgery (most definitive) | First mastectomy or last lumpectomy (if no mastectomy) within 45 days prior to diagnosis or within 365 days after dx |
Mastectomy: CPT: 19140, 19180, 19182, 19300, 19303, 19304, 19200, 19220, 19240, 19305–19307 ICD-9: 85.34, 85.36, 85.4, 85.43, 85.44, 85.45, 85.46, 85.47, 85.48, 85.35 |
|
Lumpectomy: CPT: 19120, 19125, 19126, 19160, 19301, 19162, 19302 ICD-9: 85.2, 85.20, 85.21, 85.22, 85.23 |
||
| Axillary Surgery (most definitive) | within 45 days prior to diagnosis or within 365 days after diagnosis |
Axillary lymph node dissection:
|
Sentinel lymph node biopsy:
|
||
|
No axillary surgery: if there no axillary surgery CPT code OR if no lymph nodes are examined |
||
CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, 9th revision
neoadjuvant chemotherapy was defined using the same codes with the XXXXX time frame
Determination of ACOSOG Z0011 eligibility
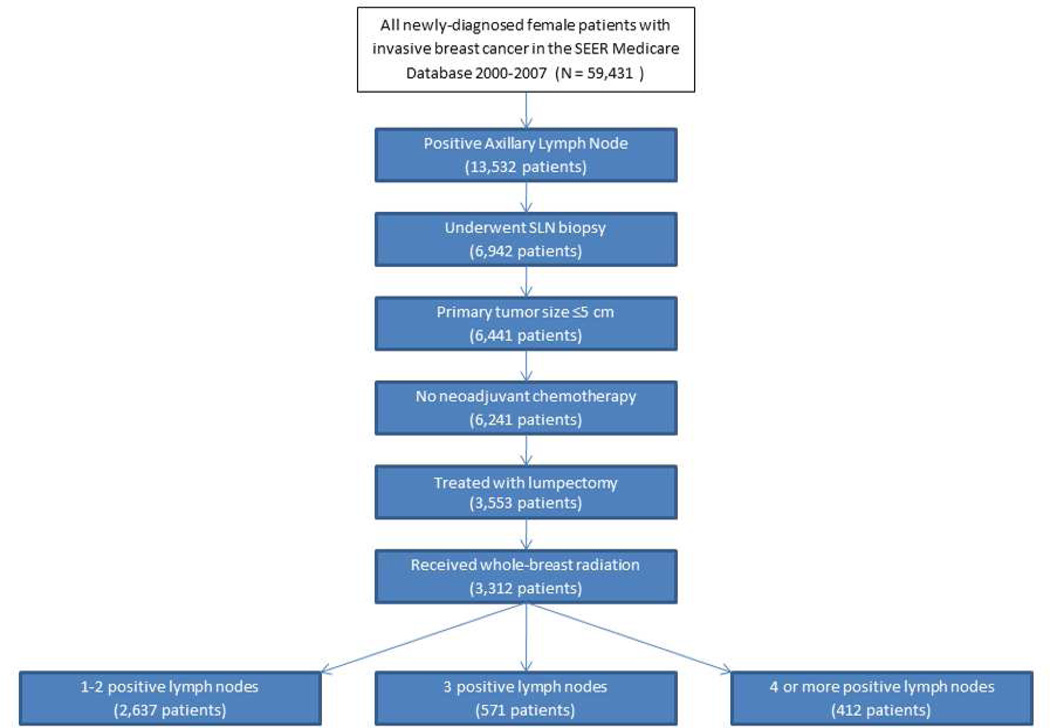
Figure 1 demonstrates how the ACOSOG Z0011 eligibility criteria were applied. To identify the proportion of patients for whom the ACOSOG Z0011 trial data can be applied, we considered only patients who underwent a SLN mapping procedure (patients with no surgical axillary staging procedure were excluded). We were unable to directly assess clinical axillary lymph node status using the SEER-Medicare database. Therefore, we used receipt of a SLN biopsy as a surrogate for clinically negative axilla; patients who did not have SLN mapping performed but had an ALND performed were assumed to have clinically evident disease at the time of presentation and therefore not eligible for ACOSOG Z0011. We excluded patients with large tumors (> 5 cm or T3/T4) or who received neoadjuvant chemotherapy. Patients who underwent lumpectomy followed by whole-breast radiation as their definitive local management were considered eligible. Finally, we examined the number of positive nodes. We are unable to distinguish within the SEER-Medicare database the number of nodes positive at the time of SLN versus the total number of nodes positive after the completion ALND. We instead categorized the number of positive nodes into 1–2, 3 or ≥4 positive lymph nodes to allow full consideration of which patients are most likely eligible for ACOSOG Z0011 (those with 1 or 2 positive lymph nodes), possibly eligible (3 positive lymph nodes) and unlikely to be eligible (≥4 positive lymph nodes).
Figure 1.
Selection of patients eligible for ACOSOG Z0011.
Analysis
The ACOSOG Z0011 inclusion criteria4,5 was applied to the overall cohort to determine the proportion of patients within the SEER Medicare database with newly diagnosed invasive breast cancer who would meet the eligibility criteria. Briefly, patients in ACOSOG Z0011 were considered eligible for the trial if they had histologically confirmed breast cancer < 5 cm in size, were clinically node negative, underwent lumpectomy with planned whole breast radiation, and had 1–2 SLN’s with metastases identified (without matting or gross extranodal extension). Descriptive statistics for demographic and clinical characteristics were generated. Actual surgical axillary management of the cohort eligible for ACOSOG Z0011 was then assessed. The characteristics of patients meeting the ACOSOG Z0011 inclusion criteria who underwent completion ALND were compared to those who forwent further axillary surgery with chi-square tests using Stata (College Station, TX). Similar data and statistics for those undergoing mastectomy that would have otherwise been eligible under ACOSOG Z0011 (if they had undergone lumpectomy) were also assessed.
Results
Of the 59,431 women >66 years of age diagnosed with invasive breast cancer from 2001 to2007, 22.8% (13,532) were node positive. Figure 1 presents the application of the ACOSOG Z0011 eligibility criteria to the node positive cohort. Of the 13,532 women with positive lymph nodes, 6,942 women underwent lymphatic mapping and were assumed to have clinically negative axilla. Fifty-one percent underwent lumpectomy, with receipt of mastectomy being the most common reason patients with a positive lymph node did not meet eligibility for ACOSOG Z0011. Overall, 2,637 patients (4.4% [2,637/59,431] of the total cohort but 38% [2,637/6,942] of patients with SLN mapping and positive nodes and 80% [2,637/3,312] of patients with SLN mapping and positive nodes who were treated with lumpectomy plus radiation) had 1or 2 positive lymph nodes and therefore met inclusion criteria for the ACOSOG Z0011 trial and could have been spared an ALND (Table 2). An additional 258 patients had 3 positive lymph nodes.
Table 2.
Elderly patients potentially eligible for ACOSOG Z0011
| Definition of Patient Cohort | N | % of cohort with 1 or 2 positive lymph nodes who are potentially eligible for ACOSOG Z0011 |
|---|---|---|
| Total cohort of elderly breast cancer patients | 59,431 | 4.4% |
| Elderly breast cancer patients, underwent SLN mapping, and have pathologically positive lymph nodes | 6,942 | 38% |
| Elderly breast cancer patients, underwent SLN mapping, have pathologically positive lymph nodes, and treated with lumpectomy + whole breast radiation | 3,312 | 80% |
Of the 2,637 patients eligible for ACOSOG Z0011, 1,214 (46%) received a completion ALND and 1,423 (54%) did not receive additional axillary surgery beyond the SLN biopsy. Table 3 presents the demographics of these two groups. Patient’s receiving ALND were more likely to be younger (p<0.001), have fewer comorbidities (p=0.05), and be more likely to receive chemotherapy (p<0.001).
Table 3.
Characteristics of Patients Eligible for ACOSOG Z0011 Based on Axillary Surgery Received
| SLN Biopsy Alone |
Completion ALND |
P value | ||
|---|---|---|---|---|
| N=1,423 | N=1,214 | |||
| Age | ||||
| 66–70 | 406 (29%) | 447 (37%) | ||
| 71–75 | 393 (27%) | 369 (30%) | p<0.001 | |
| 76–80 | 366 (26%) | 255 (21%) | ||
| 81+ | 258 (18%) | 143 (12%) | ||
| Tumor Size | ||||
| <2 cm | 923 (65%) | 763 (63%) | p=0.3 | |
| 2–5 cm | 500 (35%) | 451 (37%) | ||
| ER-PR Status | ||||
| Positive | 1003 (70%) | 841 (69%) | p=0.3 | |
| Negative | 311 (22%) | 293 (24%) | ||
| Unknown | 109 (8%) | 80 (7%) | ||
| Charlson | ||||
| Comorbidity Index | ||||
| 0 | 955 (67%) | 866 (71%) | ||
| 1 | 337 (24%) | 250 (21%) | p=0.05 | |
| 2 | 130 (9%) | 94 (8%) | ||
| Receipt of Chemotherapy | ||||
| Yes | 498 (35%) | 623 (51%) | ||
| No | 925 (65%) | 591 (49%) | p<0.001 | |
Given the high rate of SLN only in the lymph node positive lumpectomy patients, practice patterns for those patients who underwent mastectomy as the definitive breast surgery were examined to see how rates of completion ALND compared. Of the cohort of 6,241patients with a primary tumor <5 cm who underwent SLN mapping, 2,688 underwent mastectomy. One or two positive nodes were identified in 70% (n=1,881), of whom 18% received post-mastectomy radiation. Of the 1,881 patients, 877 (53%) received a completion ALND in actual practice and were more likely be younger (p<0.001) and to receive chemotherapy (p<0.001). In general, axillary surgery practices for patients undergoing mastectomy paralleled that seen for patients treated with lumpectomy and whole breast radiation.
Discussion
In this observational study of Medicare recipients, we estimated that 38% of older breast cancer patients with who underwent SLN mapping with positive lymph nodes could be spared an ALND based on ACOSOG Z0011 criteria. This is in line with other single institution experiences who have reported rates of approximately 45%.7,9 From the perspectives of these women spared an ALND and its concomitant morbidity (i.e. lymphedema, paresthesias), 17–19 ACOSOG Z0011 is clearly a practice changing trial.
However, in our population-based dataset, we also observed that 54% of ACOSOG Z0011 eligible patients were already forgoing a completion ALND in exchange for observation alone prior to 2007. Prior to the results of ACOSOG Z0011 being reported in 2010, several observational studies reported similar survival and local recurrence outcomes for patients with early stage primary tumors and a low burden of axillary disease, regardless of whether they underwent a completion ALND or not.20–28 Additional indirect evidence supporting avoidance of ALND came from two randomized controlled trials which compared ALND versus no ALND in patients with a clinically negative axilla (SLN was not performed in either study).29,30 In both trials survival and local recurrence were the same. Although these studies cannot be directly extrapolated to patients with proven nodal metastases, they further support the trend away from ALND in breast cancer and may have contributed to the patterns of care observed in our study.
What about those patients with SLN metastases who underwent mastectomy? In all reported series as well as our own study, the primary reason why patients would not meet eligibility criteria for ACOSOG Z0011 was receipt of a mastectomy as the definitive breast surgery,7,9 with one study reporting that almost 75% of patients with positive SLNs would be spared an ALND if they elected or were candidates for breast conserving therapy.7 Although patients receiving mastectomy were excluded from ACOSOG Z0011, we observed that many Medicare patients with positive lymph nodes treated with mastectomy were forgoing further axillary surgery if only 1–2 axillary lymph nodes are found to be positive (877 patients or 47%).
There is some limited data to support this practice. The recently published International Breast Cancer Study Group Trial 23-01 randomized patients with SLN micrometastases (<2 mm) to completion ALND versus observation.31 Nine percent of patients enrolled on the trial were treated with mastectomy, with no difference in breast cancer events in this very low risk subgroup, regardless of axillary surgery performed.31 A similar trend can be observed in the NSABP–B32 trial which validated the SLN concept by randomizing patients with clinically negative axilla to SLN followed by ALND versus SLN followed by ALND only if the SLN was positive. In this trial, all patients with a positive SLN underwent completion dissection. However, SLNs of patients found to be node negative underwent central review using both routine and immunohistochemical staining; occult metastases were identified in 15.9% of node negative patients. Of these patients, 107 were managed with mastectomy.1 As was observed in the IBCSG trial, no detriment in outcome was observed for these mastectomy patients with very low burden axillary disease treated with observation alone.31 Although far from definitive, data such as this provide some supporting evidence that the trend away from ALND observed in our SEER-Medicare cohort may not be resulting in poorer outcomes for these women.
There are several limitations to this study. First, our analysis of the SEER Medicare database describes the projected impact of the ACOSOG Z0011 study on the Medicare population rather than change in actual practice due to the dissemination of ACOSOG Z0011 findings. However, population level data assessing actual dissemination will not be available for several years. Second, the SEER Medicare database does not provide information on the number of positive SLN’s identified; we only know the total number of positive lymph nodes examined. Therefore, we cannot precisely identify our eligible cohort. We addressed this limitation by presenting categories of the number of positive nodes. Of the 3,312 patients who underwent SLN mapping with positive lymph nodes treated with lumpectomy and radiation, we estimated that 80% had 1–2, 8% had 3, and 12% had four or more positive lymph nodes. In our analysis, we considered those with 1–2 nodes positive to be our most conservative estimate of potential eligible patients, although this likely underestimates eligible patients as some of those patients with 3 positive nodes could be considered eligible (if some of those nodes were identified at ALND rather than SLN). Additionally, we used receipt of SLN mapping as a surrogate for clinically negative axilla. This may underestimate eligible patients if some surgeons had slow adoption of SLN biopsy as an alternative to ALND as standard of care.32 A final limitation of our study is that our analysis is limited to the Medicare population and we cannot make conclusions regarding the potential impact of ACOSOG Z0011 on younger populations. However, given that patients >65 years of age comprise more than 40% of all new breast cancer diagnoses and may be the patients most likely to have ACOSOG Z0011 data applied to them, our findings have significant clinical relevance.
Conclusion
In conclusion, we determined that ~38% of older breast cancer patients who underwent SLN mapping and were found to have positive lymph nodes would be spared an ALND in the era of ACOSOG Z0011. However, in our pre-ACOSOG Z0011 dataset, we observed that <50% of eligible older breast cancer patients were receiving a completion ALND even prior to trial results being reported, thereby dampening the true impact of this trial on clinical practice. It is noteworthy that the trend away from completion ALND in older patients with low volume axillary disease extended in our cohort to patients receiving mastectomy. Future work will need to better define the role of completion ALND for subgroups who do not meet eligibility for AOCOSOG Z0011, but for whom the risk of axillary recurrence is low, particularly those patients with low volume axillary disease treated with mastectomy.
Acknowledgements
Interpretation and reporting of these data are the sole responsibility of the authors. The efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and SEER Program in the creation of the SEER-Medicare database are acknowledged.
Disclosure of Funding: This project was funded through the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) Academic Oncologist Training Program (NIH 5K12CA087718). Further funding came from grant number P30 CA014520 from the National Cancer Institute, the Health Innovation Program, the Community-Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (grant number UL1TR0000427 from the Clinical and Translational Science Award program of the National Center for Research Resources, NIH National Center for Advancing Translational Sciences [NCATS]) and the UW School of Medicine and Public Health from The Wisconsin Partnership Program.
References
- 1.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. The lancet oncology. 2010 Oct;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. The New England journal of medicine. 2003 Aug 7;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 3.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Annals of surgery. 2005 Oct;242(4):593–599. doi: 10.1097/01.sla.0000184210.68646.77. discussion 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2011 Feb 9;305(6):569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Annals of surgery. 2010 Sep;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caudle AS, Hunt KK, Kuerer HM, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Annals of surgical oncology. 2011 Sep;18(9):2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi M, Kuerer HM, Mittendorf EA, et al. Impact of the american college of surgeons oncology group Z0011 criteria applied to a contemporary patient population. Journal of the American College of Surgeons. 2013 Jan;216(1):105–113. doi: 10.1016/j.jamcollsurg.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Annals of surgical oncology. 2012 Oct;19(10):3144–3151. doi: 10.1245/s10434-012-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guth U, Myrick ME, Viehl CT, Schmid SM, Obermann EC, Weber WP. The post ACOSOG Z0011 era: does our new understanding of breast cancer really change clinical practice? European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2012 Aug;38(8):645–650. doi: 10.1016/j.ejso.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Medical care. 1993 Aug;31(8):732–748. [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002 Aug;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med. Care. 2002 Aug;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV-3-18. [DOI] [PubMed] [Google Scholar]
- 13.Dimick JB, Osborne NH, Hall BL, Ko CY, Birkmeyer JD. Risk adjustment for comparing hospital quality with surgery: how many variables are needed? Journal of the American College of Surgeons. 2010 Apr;210(4):503–508. doi: 10.1016/j.jamcollsurg.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Cancer AJCo. AJCC Cancer Staging Manual. 6th edition. Philadelphia, PA: Lippincott Raven Publishers; 2002. [Google Scholar]
- 17.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Annals of surgical oncology. 2006 Apr;13(4):491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. Journal of surgical oncology. 2010 Aug 1;102(2):111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Annals of surgical oncology. 2008 Jan;15(1):262–267. doi: 10.1245/s10434-007-9593-3. [DOI] [PubMed] [Google Scholar]
- 20.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Annals of surgical oncology. 2010 Oct;17(Suppl 3):343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setton J, Cody H, Tan L, et al. Radiation field design and regional control in sentinel lymph node-positive breast cancer patients with omission of axillary dissection. Cancer. 2012 Apr 15;118(8):1994–2003. doi: 10.1002/cncr.26504. [DOI] [PubMed] [Google Scholar]
- 22.Fan YG, Tan YY, Wu CT, et al. The effect of sentinel node tumor burden on non-sentinel node status and recurrence rates in breast cancer. Annals of surgical oncology. 2005 Sep;12(9):705–711. doi: 10.1245/ASO.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Yegiyants S, Romero LM, Haigh PI, DiFronzo LA. Completion axillary lymph node dissection not required for regional control in patients with breast cancer who have micrometastases in a sentinel node. Archives of surgery (Chicago, Ill : 1960) 2010 Jun;145(6):564–569. doi: 10.1001/archsurg.2010.84. [DOI] [PubMed] [Google Scholar]
- 24.Fournier K, Schiller A, Perry RR, Laronga C. Micrometastasis in the sentinel lymph node of breast cancer does not mandate completion axillary dissection. Annals of surgery. 2004 Jun;239(6):859–863. doi: 10.1097/01.sla.0000128302.05898.a7. discussion 863–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langer I, Guller U, Viehl CT, et al. Axillary lymph node dissection for sentinel lymph node micrometastases may be safely omitted in early-stage breast cancer patients: long-term outcomes of a prospective study. Annals of surgical oncology. 2009 Dec;16(12):3366–3374. doi: 10.1245/s10434-009-0660-9. [DOI] [PubMed] [Google Scholar]
- 26.Meretoja TJ, Vironen JH, Heikkila PS, Leidenius MH. Outcome of selected breast cancer patients with micrometastasis or isolated tumor cells in sentinel node biopsy and no completion axillary lymph node dissection. Journal of surgical oncology. 2010 Sep 1;102(3):215–219. doi: 10.1002/jso.21608. [DOI] [PubMed] [Google Scholar]
- 27.Pernas S, Gil M, Benitez A, et al. Avoiding axillary treatment in sentinel lymph node micrometastases of breast cancer: a prospective analysis of axillary or distant recurrence. Annals of surgical oncology. 2010 Mar;17(3):772–777. doi: 10.1245/s10434-009-0804-y. [DOI] [PubMed] [Google Scholar]
- 28.Cohen ME, Dimick JB, Bilimoria KY, Ko CY, Richards K, Hall BL. Risk adjustment in the American College of Surgeons National Surgical Quality Improvement Program: a comparison of logistic versus hierarchical modeling. Journal of the American College of Surgeons. 2009 Dec;209(6):687–693. doi: 10.1016/j.jamcollsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Rudenstam CM, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10–93. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Dec;24(3):337–344. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 30.Martelli G, Boracchi P, Ardoino I, et al. Axillary dissection versus no axillary dissection in older patients with T1N0 breast cancer: 15-year results of a randomized controlled trial. Annals of surgery. 2012 Dec;256(6):920–924. doi: 10.1097/SLA.0b013e31827660a8. [DOI] [PubMed] [Google Scholar]
- 31.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. The lancet oncology. 2013 Apr;14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderveen KA, Paterniti DA, Kravitz RL, Bold RJ. Diffusion of surgical techniques in early stage breast cancer: variables related to adoption and implementation of sentinel lymph node biopsy. Annals of surgical oncology. 2007 May;14(5):1662–1669. doi: 10.1245/s10434-006-9336-x. [DOI] [PubMed] [Google Scholar]