Abstract
Burkholderia mallei and B. pseudomallei are Gram-negative pathogenic bacteria, responsible for the diseases glanders and melioidosis, respectively. Furthermore, there is currently no vaccine available against these Burkholderia species. In this study, we aimed to identify protective proteins against these pathogens. Immunization with recombinant B. mallei Hcp1 (type VI secreted/structural protein), BimA (autotransporter protein), BopA (type III secreted protein), and B. pseudomallei LolC (ABC transporter protein) generated significant protection against lethal inhaled B. mallei ATCC23344 and B. pseudomallei 1026b challenge. Immunization with BopA elicited the greatest protective activity, resulting in 100% and 60% survival against B. mallei and B. pseudomallei challenge, respectively. Moreover, sera from recovered mice demonstrated reactivity with the recombinant proteins. Dendritic cells stimulated with each of the different recombinant proteins showed distinct cytokine patterns. In addition, T cells from immunized mice produced IFN-γ following in vitro re-stimulation. These results indicated therefore that it was possible to elicit cross-protective immunity against both B. mallei and B. pseudomallei by vaccinating animals with one or more novel recombinant proteins identified in B. mallei.
Keywords: Burkholderia, B. mallei, B. pseudomallei, vaccine, subunit vaccination, intranasal infection
Introduction
Vaccines are the most efficacious and cost-effective means of protecting human and animal populations from infection. At present, there are no approved vaccines available for use in protecting animals or humans against the Gram-negative bacterial pathogens Burkholderia mallei and B. pseudomallei. Therefore, we sought to develop and evaluate vaccines that might be used to generate cross-protective immunity against both pathogens.
B. mallei is a non-motile bacterium responsible for glanders. This disease mainly affects horses, which are considered to be the natural reservoir for infection, although mules and donkeys are also susceptible (1). Humans are accidental hosts of B. mallei following prolonged and close contact with infected animals. B. mallei infects humans by entering through open wounds and surfaces of the eyes or nose. Symptoms of glanders are dependent on the route of infection (2). B. pseudomallei are motile bacteria causing melioidosis (3). Melioidosis is a life-threatening disease that is mainly acquired through skin inoculation or pulmonary contamination, although other routes have been documented. This saprophyte inhabitant of soil environments is mainly encountered in Southeast Asia and Northern Australia, but is sporadically isolated in subtropical and temperate countries (4).
Both Burkholderia species are highly pathogenic and are classified as such in list B by the Centers for Disease Control and Prevention (5). Burkholderia infections are difficult to treat with antibiotics and there are several reports that indicate it is feasible to protect against melioidosis, at least in animal models of disease, with non-living vaccines (6). There has also been some progress in identifying partially protective subunits. Passively administered antisera raised against flagellin, polysaccharide, or conjugates of polysaccharide and flagellin, protect diabetic rats against challenge with B. pseudomallei (7–9). However, B. mallei are not motile and do not produce flagella. Moreover, the ability of flagellin to induce protection against an aerosol, or intranasal challenge has not been reported. Therefore, we assessed flagellin as a potential candidate for inclusion in a Burkholderia vaccine and found it unsuitable (our unpublished data). In contrast, all of the current evidence indicates that other surface-expressed or secreted proteins are immunogenic and structural similarity exists between the proteins in B. pseudomallei and B. mallei (10–11). In this study, we aimed to identify Burkholderia protective proteins that could be administered in vaccines to generate cross-protective immunity against both B. mallei and B. pseudomallei. We hypothesize that cross-protection is possible based on the similarities in antigenic composition and mechanisms of protection between these organisms, and if this is true, development of a single vaccine which stimulates T-cell and antibody responses against melioidosis and glanders-producing bacterial agents is feasible. If cross-protective immunity is observed, then it may be possible to develop a single vaccine capable of generating protection against both melioidosis and glanders.
2. Recombinant protein expression and purification
Bioinformatics analysis of target sequences was used to indicate the presence (or absence) of an N-terminal secretion sequence, transmembrane domains and homology to published crystal structures. The programs used were SignalP v.3.0, TMHMM v.2.0 and PHYRE v.0.2, respectively (12–14). DNA sequences coding for B. mallei proteins BopA (BMA_A1521; AA 23 – 512), BimA (BMA_A0749; residues 19 – 265), Hcp1 (BMA_A0742; residues 1 – 169) and the B. pseudomallei protein LolC (BPSL2277; residues 44 – 266) (15) were cloned into the pET28a (+) expression vector (Novagen). Primers were designed to PCR-amplify and clone the selected sequences in frame with a C-terminal 6x Histidine tag, for all four targets. Expand high fidelity DNA polymerase (Roche) was used to amplify targets from B. mallei ATCC 23344 or B. pseudomallei K96243 genomic DNA. Once ligated into pET28a (+), plasmid DNA was electroporated into Escherichia coli DH5α. Cloned sequences were verified by DNA sequencing, using T7 promoter/terminator oligonucleotide primers.
Target protein expression in E. coli (λDE3) Rosetta was induced by growth in Overnight Express instant TB medium (Novagen) for 18 – 20 h. Bacterial pellets were lysed using 10x CelLytic B (Sigma), and 6x His-tagged proteins were purified by Ni2+ affinity chromatography. Purified proteins were dialyzed against two changes of 10 mM Hepes/150 mM NaCl, pH 7.4, aliquoted and stored at −80 °C. Protein concentrations were determined using the BCA kit (Pierce) using bovine serum albumin (BSA) as a standard, and sample purity assessed by SDS-PAGE.
3. Vaccination and challenge with B. pseudomallei or B. mallei
To evaluate the potential of Burkholderia surface expressed or secreted proteins to generate protective immunity, the purified recombinant proteins were used individually or in combination to vaccinate mice via the intranasal (i.n.) route. For immunization of mice against B. mallei challenge, 6–8 week old female BALB/c mice (n = 8 per group) were primed with 10 μg of recombinant proteins mixed with adjuvant (12.5 μg of CpG oligodeoxynucleotide (ODN) 2395 (Coley Pharmaceuticals) and mixed with 12.5 μg immune-stimulating complex (ISCOM) AbISCO 100 (Isconova AB), followed by a 2 week boost of 5 μg recombinant proteins with adjuvant. Four weeks post-boost, animals were infected by intranasal inoculation with 2 LD50 of B. mallei ATCC 23344 administered i.n. to anesthetized mice. Control animals were vaccinated with non-specific protein (Bovine Serum Albumin, BSA) and adjuvant. Animals were observed closely following challenge and euthanized immediately when pre-determined endpoints were reached and these time points were used to calculate survival times. All animal studies were approved by the Institutional Animal Care and Use Committee at UTMB.
Following challenge with B. mallei, 12.5% of control animals survived for > 21 days (Figure 1A). In contrast, survival percentages were significantly increased to 100% up to 21 days post-infection in mice vaccinated with recombinant BimA or BopA. The surviving animals were euthanized at day 21 post-challenge, and the lungs and spleens were homogenized and bacterial counts determined. In all the surviving animals, B. mallei were not recovered from the lungs. However, B. mallei were recovered from the spleens of all surviving animals (data not shown).
Fig. 1.
Survival of BALB/c mice immunized with different recombinant proteins and challenged with B. mallei ATCC 23344 and B. pseudomallei 1026b.
BALB/c mice were challenged i.n. with 2 LD50 B. mallei 4 weeks following intranasal vaccination with BimA (n= 2), BopA (n=5), Combo (n=5), LolC (n=6), Hcp1 (n=8) or Control (n= 8). BopA- and BimA-vaccinated animals resulted in 100% survival up to 21 days post-challenge. (B) BALB/c mice (n = 15, pooled data from 3 separate experiments) were immunized 3 times with the indicated antigens, then challenged i.n. with 2 LD50 B. pseudomallei strain 1026b and survival times determined,.
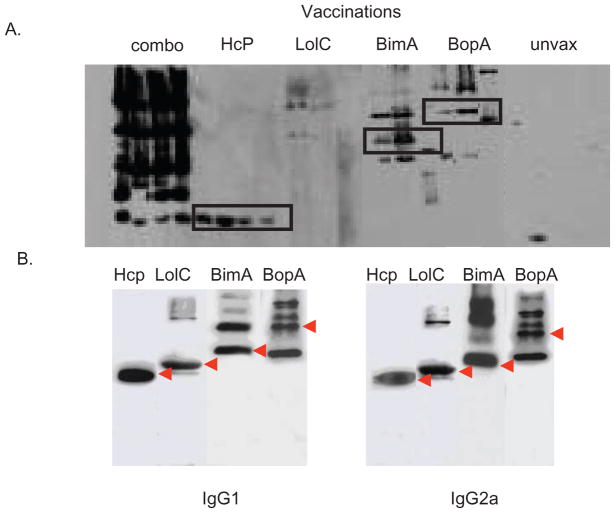
To determine whether antibodies from infected mice reacted with the recombinant Burkholderia proteins, serum was obtained from surviving animals. These sera were then tested for recognition of the purified recombinant Burkholderia antigens Hcp1, BimA, BopA and LolC by Western blot. Serum from infected mice recognized each of the recombinant antigens tested except for the LolC protein (Figure 2A) and were recognized by both IgG1 and IgG2a isotypes (Figure 2B).
Fig. 2.
(A). B. mallei antibody response post-vaccination. Western blots were performed on sera collected 2 weeks post-boost to determine IgG reactivity. Mice vaccinated with recombinant BopA, BimA, LolC and Hcp1, individually or in combination (combo), demonstrated response to all proteins except LolC. Individually vaccinated mice produced a robust humoral response, although LolC was lacking. (B) Isotype-specific responses to the vaccine candidates (IgG1 and IgG2a) detected from vaccinated and challenged mice.
Experiments were also conducted to determine whether the Burkholderia recombinant antigens were also capable of generating protective immunity against B. pseudomallei challenge (Figure 1B). For these experiments BALB/c mice, (n = 5 per group) were primed by i.n. inoculation with two adjuvant systems and immunized with 2 μg of the purified recombinant BimA, BopA, or LolC proteins given with CLDC adjuvant (cationic liposome-DNA complex), and then boosted 2 weeks later and again 2 weeks after that. The adjuvant used for these studies consisted of cationic liposome-DNA complexes, as reported previously (16) for use in non-specific immunotherapy of B. pseudomallei. Controls consisted of mice administered CLDC adjuvant alone, diluent only or BopA antigen alone. Two weeks after the last immunization, mice were subjected to lethal i.n. challenge with 2 LD50 of B. pseudomallei strain 1026b, as described previously (17). Survival times were determined as noted above, and all the B. pseudomallei animal challenge studies were approved by the Institutional Animal Care and Use Committee at Colorado State University.
Our data indicate that the vaccine candidates protected the animals from an initial, acute infection, but failed to confer sterilizing immunity. Our future studies are focusing on the identification of the immunogenic domains in the proteins coupled with optimization in the vaccination strategies to develop a fully protective vaccine.
We observed that immunization separately or as a combination of each of the four recombinant Burkholderia antigens conferred at least 75% protection against B. mallei infection at 21 days compared to control mice (Figures 1A and 1B). Notably, one of the vaccine antigens (BopA) also conferred significant 60% longer term (> 60 days) protection against B. pseudomallei challenge. Furthermore, when surviving BopA-vaccinated mice were sacrificed and lung, spleen, and liver tissues plated for detection of B. pseudomallei, 25% of the surviving animals were sterile, at least within the limits of detection of the organ culture (typically 50 CFU/organ).
4. ELISA assay for humoral responses to vaccination
Blood was removed from the orbital veins of immunized mice 2 days post-boost. The blood was allowed to clot at room temperature prior to centrifugation at 5,000 × g. Serum was collected and stored at −20 °C. The recombinant Hcp1-, LolC-, BimA- and BopA-specific protein responses were determined by ELISA (Table 1). Briefly, microtiter plates were coated with 5 μg/ml of the appropriate recombinant protein in PBS overnight. Non-specific binding was blocked using 1% (w/v) ovalbumin in PBS (OVA-PBS) for 1 h at RT. The plates were washed three times using 0.05% (v/v) Tween 20 in PBS, and appropriate dilutions of sera in OVA-PBS were added in triplicate and incubated for 2 h at 37 °C. Following the washes, biotinylated-rat-anti-mouse IgG1or IgG2a (BD Biosciences) diluted in OVA-PBS was added and incubated for 2 h at 37 °C. Next, HRP-conjugated Streptavidin (BD Biosciences) was added and incubated for 25 min at RT. The substrate ABTS was added and the absorbance at 405 nm was measured. Antibody concentrations (in μg/ml) were calculated from standard curves generated with IgG1 or IgG2a specific antibodies.
Table 1.
Immune response to Recombinant Burkholderia Proteins
| Protein | IgG1a (μg/ml) | IgG2aa (μg/ml) |
|---|---|---|
| Hcp1 | 95 | 40 |
| LolC | 0.104 | 0.902 |
| BimA | 2,970 | 5,700 |
| BopA | 2,070 | 6,080 |
The IgG1 and IgG2a responses were analyzed by ELISA using sera removed from immunized and infected mice.
5. Discussion
Immunization with recombinant LolC, BimA, Hcp1HCP and BopA proteins provided significant protection against B. mallei ATCC 23344 and B. pseudomallei 1026b (Figures 1A and B), in which B. mallei BopA gave the best results. The combination of all subunits for protection from B. mallei may have some utility as a combined vaccine, although not remarkably better than BopA alone. The serological results suggest that optimal level of Th1 (IgG2a) and Th2 (IgG1) responses are important for protection in B. mallei infection. An interesting observation is that in control group mice, which received only CpG2395 and ISCOM, we have observed over 78% survival, whereas in a previous study, mice receiving only ISCOM had ~12% survival after infection with 2 LD50 of B. mallei ATCC 23344, indicating that CpG2395 itself also offers protection (data not shown). There are several reports showing immune-enhancing activity of CpG (18–24). Consistent with this study, all future studies will be repeated using a more prolonged time to challenge at 4 weeks to reduce background protection offered by CpG2395, and a control group without CpG treatment will be included as a control as was done in these studies.
Acknowledgments
This work was supported by NIH National Institute of Allergy and Infectious Diseases, Western Regional Center for Biodefense and Emerging Infections (D.M.E., A.G.T and K.A.B) and a fellowship award to G.C.W. from the UTMB Sealy Center for Vaccine Development. The work was also supported by NIH-NIAID grant U54 AI065357 (KP, AD, SD).
References
- 1.Neubauer H, Sprague LD, Zacharia R, Tomaso H, Al Dahouk S, Wernery R, et al. Serodiagnosis of Burkholderia mallei infections in horses: state-of-the-art and perspectives. Journal of Veterinary Medicine Series B. 2005;52:201–5. doi: 10.1111/j.1439-0450.2005.00855.x. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan A, Kraus CN, DeShazer D, Becker PM, DDJ, Spacek L, et al. Glanders in a military research microbiologist. N Engl J Med. 2001;345:256–8. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 3.Dance DAB. Melioidosis - the tip of the iceberg. Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone R. Infectious disease. Racing to defuse a bacterial time bomb. Science. 2007;317:1022–4. doi: 10.1126/science.317.5841.1022. [DOI] [PubMed] [Google Scholar]
- 5.Horn JK. Bacterial agents used for bioterrorism. Surgical Infections. 2003;4:281–7. doi: 10.1089/109629603322419625. [DOI] [PubMed] [Google Scholar]
- 6.Nelson M, Prior JL, Lever MS, Jones HE, Atkins TP, Titball RW, et al. Evaluation of lipopolysaccharide and capsular polysaccharide as subunit vaccines against experimental melioidosis. J Med Microbiol. 2004;53:1177–82. doi: 10.1099/jmm.0.45766-0. [DOI] [PubMed] [Google Scholar]
- 7.Brett P, Mah D, Woods D. Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect Immun. 1994;62:1914–9. doi: 10.1128/iai.62.5.1914-1919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brett PJ, Woods DE. Structural and immunological characterization of Burkholderia pseudomallei O-polysaccharide-flagellin protein conjugates. Infect Immun. 1996;64:2824–8. doi: 10.1128/iai.64.7.2824-2828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan LE, Wong SE, Woods D, Dance D, Chaowagul W. Passive protection of diabetic rats with antisera specific for the polysaccharide portion of the lipopolysaccharide isolated from Pseudomonas pseudomallei. Can J Infect Dis. 1994;5:170–8. doi: 10.1155/1994/856850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitlock GC, Estes DM, Torres AG. Glanders: off to the races with Burkholderia. FEMS Microbiol Lett. 2007;277:115–22. doi: 10.1111/j.1574-6968.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitlock GC, Estes DM, Young GM, Young B, Torres AG. Construction of a reporter system to study Burkholderia mallei type III secretion and identification of the BopA effector protein function in intracellular survival. Transactions of the Royal Society of Tropical Medicine & Hygiene. 2008;102(Suppl 1):S127–33. doi: 10.1016/S0035-9203(08)70029-4. [DOI] [PubMed] [Google Scholar]
- 12.Bendtsen JD, von Heijne G, Brunak S, Bendtsen JD, Nielsen H, et al. Improved prediction of signal peptides: SignalP 3.0. Journal of Molecular Biology. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of Molecular Biology. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 14.Kelley LA, Sternberg MJ, Kelley LA, Sternberg MJE. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protocols. 2009;4:363–71. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 15.Harland DN, Chu K, Haque A, Nelson M, Walker NJ, Sarkar-Tyson M, et al. Identification of a LolC homologue in Burkholderia pseudomallei, a novel protective antigen for melioidosis. Infection & Immunity. 2007;75:4173–80. doi: 10.1128/IAI.00404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176:7335–45. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 17.Goodyear A, Kellihan L, Bielefeldt-Ohmann H, Troyer R, Propst K, Dow S, et al. Protection from pneumonic infection with burkholderia Burkholderia species by inhalational immunotherapy. Infection & Immunity. 2009;77:1579–88. doi: 10.1128/IAI.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y-S, Hsiao Y-S, Lin H-H, Liu Y, Chen Y-L. CpG-modified plasmid DNA encoding flagellin improves immunogenicity and provides protection against Burkholderia pseudomallei infection in BALB/c mice. Infection & Immunity. 2006;74:1699–705. doi: 10.1128/IAI.74.3.1699-1705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elvin SJ, Healey GD, Westwood A, Knight SC, Eyles JE, Williamson ED. Protection against heterologous Burkholderia pseudomallei strains by dendritic cell immunization. Infection & Immunity. 2006;74:1706–11. doi: 10.1128/IAI.74.3.1706-1711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature Reviews Drug Discovery. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 21.Utaisincharoen P, Kespichayawattana W, Anuntagool N, Chaisuriya P, Pichyangkul S, Krieg AM, et al. CpG ODN enhances uptake of bacteria by mouse macrophages. Clinical & Experimental Immunology. 2003;132:70–5. doi: 10.1046/j.1365-2249.2003.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Advanced Drug Delivery Reviews. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Waag DM, McCluskie MJ, Zhang N, Krieg AM. A CpG oligonucleotide can protect mice from a low aerosol challenge dose of Burkholderia mallei. Infection & Immunity. 2006;74:1944–8. doi: 10.1128/IAI.74.3.1944-1948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wongratanacheewin S, Kespichayawattana W, Intachote P, Pichyangkul S, Sermswan RW, Krieg AM, et al. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infection & Immunity. 2004;72:4494–502. doi: 10.1128/IAI.72.8.4494-4502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]