Abstract
OBJECTIVE
To examine the effect of prostate volume, number of biopsy cores, and American Urological Association symptom score (AUASS) for prostate cancer risk assessment among men receiving finasteride in the Prostate Cancer Prevention Trial.
METHODS
Data from 4509 men on the finasteride arm of the Prostate Cancer Prevention Trial who were on treatment at the time of their AUASS and prostate-specific antigen (PSA) measurement before biopsy were included in multivariable logistic regression analyses.
RESULTS
Six hundred eighty-two (15.1%) participants had prostate cancer; 257 (37.7%) of these had high-grade disease. For prostate cancer risk, the model included PSA (odds ratio corresponding to a 2-fold increase in PSA: 2.70; P <.0001), digital rectal examination (2.53; P <.0001), age (1.03; P = .001), and prostate volume (odds ratio 0.54 for a 2-fold increase in volume; P <.0001). For high-grade disease, PSA (3.39; P <.0001), digital rectal examination (2.75; P <.0001), age (1.05; P = .001), and volume (0.55; P <.0001) were statistically significant. AUASS was not statistically significant in any of the models that included prostate volume, but was in models in which volume was not included. The number of biopsy cores did not significantly improve risk assessment in any of the models considered.
CONCLUSION
Although in the general population, obtaining a cancer diagnosis is improved by assessing prostate volume and increasing the number of biopsy cores, neither steps are required in men receiving finasteride. Obtaining fewer biopsy cores in men receiving finasteride preserves biopsy sensitivity and will likely reduce cost and morbidity. UROLOGY 82: 1076–1082, 2013.
The Prostate Cancer Prevention Trial (PCPT) was a 7-year double-blind randomized trial of the chemopreventive effects of finasteride (5 mg/day) on prostate cancer; 18,882 men aged 55 years or older with a prostate-specific antigen (PSA) level of 3.0 ng/mL or lower, a normal digital rectal examination (DRE), and no previous diagnosis of prostate cancer were randomized.1 A unique feature of the trial was that in addition to the recommendation for prostate biopsy based on a PSA >4.0 ng/mL or an abnormal DRE, all cancer-free participants were recommended to undergo an end-of-study biopsy after 7 years of participation. Initial analyses revealed higher than anticipated prostate cancer and high-grade cancer (Gleason grade ≥7) rates than previously anticipated and no lower PSA cutoff below which prostate cancer could not be detected.2 Because end-of-study biopsies were performed regardless of PSA and DRE, the PCPT allowed comprehensive risk assessment of multiple factors affecting biopsy results, making it the biopsy cohort most immune to ascertainment bias. We found the statistically significant predictors of prostate cancer to include PSA, DRE, first-degree family history, and history of a previous negative biopsy (the latter reducing risk) and for high-grade cancer, the same risk factors minus family history but including age and African American race.3 In a subsequent assessment of men in the finasteride group, similar effects were observed, resulting in the online posting of risk calculators for prostate cancer and high-grade disease for men older than 55 years with no previous diagnosis of prostate cancer, including men receiving finasteride.4
More recently, recognizing that the study protocol of a 6-core biopsy might under-sample the prostate compared with the more common current practice of 10-12 cores and the growing evidence that prostate volume might affect cancer detection, we undertook an analysis of the effect of the number of biopsy cores, prostate volume, and the American Urological Association symptom score (AUASS – a validated measure of outlet symptoms related to benign prostatic hyperplasia [BPH]) on prostate cancer detection in the placebo arm of the PCPT.5 We found that increasing the number of biopsy cores significantly improved detection of prostate cancer and high-grade cancer at biopsy; similarly, measurement of prostate volume significantly enhanced risk assessment. AUASS was not independently associated with detection after adjustment for prostate volume, but was independently predictive if volume was not included. Commonly used to treat patients with BPH, the 5 alpha-reductase inhibitor finasteride reduces prostate volume and approximately halves PSA. In previous studies, we found that despite a significant reduction in risk of prostate cancer with finasteride, the performance of prostate biopsy is significantly improved in men receiving this medication, presumably because of the reduction in prostate volume.6 Given this effect and our previous finding of the need for an increased number of biopsy cores to maintain high sensitivity for detection of high-grade prostate cancer, we herein describe our assessment of this phenomenon in men receiving finasteride.
MATERIALS AND METHODS
Participants
A total of 4509 PCPT participants randomized to finasteride with PSA, DRE, and AUASS measured within 1 year before biopsy, PSA ≤10 ng/mL, and who were on finasteride at PSA measurement were selected for analysis. The AUASS includes 7 questions related to urinary frequency and urgency associated with BPH; each of these questions ranges from 0 (no symptoms) to 5 (highly symptomatic) for a total score ranging from 0 to 35. All men provided written informed consent, and study procedures were approved by local institutional review boards for each participating study site.
Statistical Analyses
Characteristics were compared between patients with and without cancer using the 2-sample Wilcoxon test for continuous measures and the Chi-square test for categorical measures. Spearman’s rank correlation was used to assess the correlation between prostate volume and AUASS.
For multivariable modeling, PSA was log-base-2 (log2)-transformed so that the resulting odds ratio would be interpretable as the change in odds of outcome for a 2-fold increase in PSA, that is, the same odds ratio would denote the increase in risk of prostate cancer for an increase in PSA from 0.5 to 1 ng/mL or from 5 to 10 ng/mL. Volume and PSA density, a derived risk factor computed as the ratio of PSA to volume, were log2-transformed. Participants missing volume and/or number of cores were excluded from analyses involving volume and number of cores. Number of biopsy cores, AUASS, and age were treated as continuous covariates. All possible combinations and interactions of risk factors were considered for separate prediction models for any prostate cancer and high-grade disease. For both cancer endpoints, first only volume and number of cores were included in addition to the PCPT risk factors in the multivariable models. A second set of models was developed for cases in which prostate volume was not available but AUASS was available. Because the number of biopsy cores is typically fixed by institutional practices and not discussed with the patient, it was similarly excluded from the set of second models. Optimal models were selected using the Bayesian Information Criterion. It should be noted that none of the optimal models contained 2-way interactions so only main effects were reported.
RESULTS
Of the 4509 PCPT finasteride arm participants, 682 (15.1%) participants had prostate cancer and 257 (5.7% of participants, 37.7% of cancers) high-grade disease. Compared with men with negative biopsies, men diagnosed with prostate cancer were more likely to be African American, have an abnormal DRE, a positive family history, a higher PSA, and a greater number of biopsy cores (all P values <.05; Table 1). The proportion of missing volumes was 16.3% among positive biopsy cases compared with 11.4% among negative biopsies (P value = .0004); the proportion with missing number of cores was 4.3% and 0.9%, respectively (P value <.0001). There was no difference between positive and negative biopsies in terms of prostate volume, AUASS, history of a previous biopsy, and age (all P values >.05). The correlation between AUASS and prostate volume (0.084) was very low.
Table 1.
Characteristics of the 4509 Prostate Cancer Prevention Trial finasteride arm participants used for the analysis
| Characteristic | Biopsy Negative (n = 3827) |
Biopsy Positive (n = 682) |
|---|---|---|
| Age (y) | ||
| Mean (SD) | 69.7 (5.5) | 69.9 (5.9) |
| Range | 56-92 | 56-89 |
| Race n (%)* | ||
| White | 3648 (95.4) | 640 (93.8) |
| African American | 129 (3.4) | 37 (5.4) |
| Other | 49 (1.3) | 5 (0.7) |
| Previous biopsy n (%) | ||
| Never | 3326 (86.9) | 574 (84.2) |
| At least 1 | 501 (13.1) | 108 (15.8) |
| DRE, n (%)* | ||
| Normal | 3509 (91.7) | 536 (78.6) |
| Abnormal | 318 (8.3) | 146 (21.4) |
| Family history, n (%)* | ||
| No | 3248 (84.9) | 536 (78.6) |
| Yes | 579 (15.1) | 146 (21.4) |
| PSA (ng/mL)* | ||
| Mean (SD) | 0.7 (0.6) | 1.5 (1.4) |
| Range | 0.3-8.9 | 0.3-9.6 |
| TRUS volume (mL) | ||
| Mean (SD) | 27.0 (11.6) | 27.1 (14.4) |
| Range | 10-155.1 | 10.3-239 |
| Unknown n (%) | 437 (11.4) | 111 (16.3) |
| No. of biopsy cores (%)* | ||
| 1-5 | 25 (0.7) | 12 (1.8) |
| 6 | 3105 (81.1) | 490 (71.9) |
| 7-10 | 491 (12.8) | 112 (16.4) |
| 11-17 | 171 (4.5) | 39 (5.7) |
| Unknown | 35 (0.9) | 29 (4.3) |
| AUASS | ||
| Mean (SD) | 7.4 (5.4) | 7.3 (5.4) |
| Range | 0-32 | 0-31 |
| AUASS, n (%) | ||
| 0-9 | 2722 (71.1) | 492 (72.1) |
| 10-19 | 978 (25.6) | 166 (24.3) |
| 20-29 | 123 (3.2) | 23 (3.4) |
| 30-35 | 4 (0.1) | 1 (0.2) |
| Reason for biopsy (%)* | ||
| Interim (or for cause) | 494 (12.9) | 328 (48.1) |
| End-of-study | 3331 (87.1) | 354 (51.9) |
| Gleason grade (%) | ||
| ≤4 | – | 16 (2.4) |
| 5 | – | 53 (7.8) |
| 6 | – | 347 (50.9) |
| 7 | – | 179 (26.3) |
| ≥8 | – | 78 (11.4) |
| Unknown | – | 9 (1.3) |
AUASS, American Urological Association symptom score; DRE, digital rectal examination; PSA, prostate-specific antigen; SD, standard deviation; TRUS, transrectal ultrasound.
P value <.05 for difference between biopsy negative and positive.
For each cancer endpoint (overall prostate cancer and high-grade disease) 2 risk models were developed: (1) clinical models incorporating prostate volume and number of cores, and (2) AUA symptom score models that did not include prostate volume and number of cores in an attempt to provide a risk model available in the absence of variables commonly not measured at biopsy.
Table 2 lists all the previous PCPT risk factors, along with the new factors considered in this analysis. PSA, DRE, and age were statistically significant in all models with increasing risk of prostate cancer and high-grade disease for an increased PSA (an increase in odds between 2.56- and 3.24-fold for a doubling of PSA), abnormal DRE (an increase in odds between 2.53- and 3.06-fold for an abnormal DRE), and older age (an increase in odds between 1.02- and 1.05-fold for each year increase in age). African American race and family history were not selected for inclusion in any models by the Bayesian Information Criterion criterion. Previous biopsy only remained in the model incorporating AUASS for prediction of overall prostate cancer, with a decrease in odds of cancer (odds ratio = 0.04; P value = .0006) for participants with a history of a previous negative biopsy. The number of biopsy cores was not statistically significant in any of the models considered and so was not chosen for inclusion in the optimal models. AUASS was not statistically significant in any models that already included prostate volume, but was selected in optimal models that did not include volume. The effect of AUASS on risk, however, was minor, with P-values close to .05 and a unit-increase in AUASS associated with a decrease in the odds of overall and high-grade disease of only 2% and 3%, respectively. Volume was highly statistically significant for overall and high-grade prostate cancer, with an approximate halving of the odds of either endpoint for a doubling of volume. PSA density did not appear in any of the optimal models incorporating prostate volume as an adjunct to or in lieu of the risk factors PSA and volume.
Table 2.
Odds ratios, 95% confidence intervals, and P values for the optimal multivariable models
| Overall Cancer |
High-grade Cancer |
|||
|---|---|---|---|---|
| Variable | All Predictors Allowed | Volume, Number of Cores Not Allowed |
All Predictors Allowed | Volume, Number of Cores Not Allowed |
| PSA* | 2.70 | 2.56 | 3.39 | 3.24 |
| 2.45-2.97 | 2.35-2.78 | 2.94-3.90 | 2.85-3.67 | |
| P <.0001 | P <.0001 | P <.0001 | P <.0001 | |
| DRE | 2.53 | 2.89 | 2.75 | 3.06 |
| 1.93-3.33 | 2.27-3.69 | 1.87-4.04 | 2.20-4.27 | |
| P <.0001 | P <.0001 | P <.0001 | P <.0001 | |
| Age | 1.03 | 1.02 | 1.05 | 1.05 |
| 1.01-1.05 | 1.00-1.04 | 1.02-1.07 | 1.02-1.07 | |
| P = .001 | P = .02 | P = .001 | P = .0003 | |
| Race (African American) | – | – | – | – |
| Previous negative biopsy | 0.64 | |||
| – | 0.49-0.82 | – | – | |
| P = .0006 | ||||
| Family history | – | – | – | – |
| Volume* | 0.54 | 0.55 | ||
| 0.45-0.64 | Not allowed | 0.41-0.72 | Not allowed | |
| P <.0001 | P <.0001 | |||
| Number biopsy cores | – | Not allowed | – | Not allowed |
| AUASS | 0.98 | 0.97 | ||
| – | 0.97-1.00 | – | 0.95-1.00 | |
| P = .04 | P = .03 | |||
Abbreviations as in Table 1.
Corresponding to a change in odds for a doubling of the variable; – denotes not included in the model with lowest Bayesian Information Criterion.
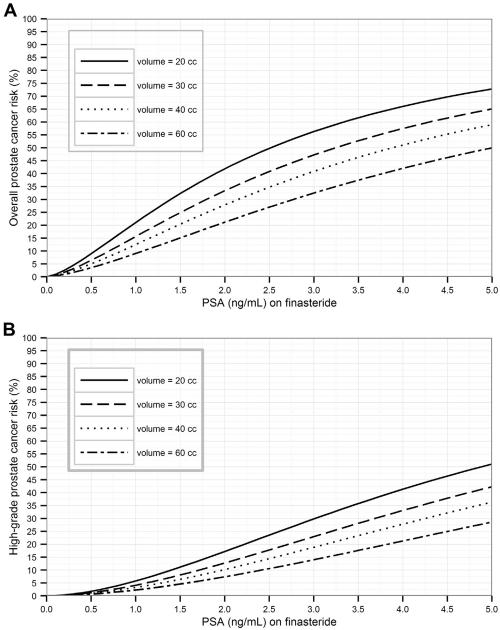
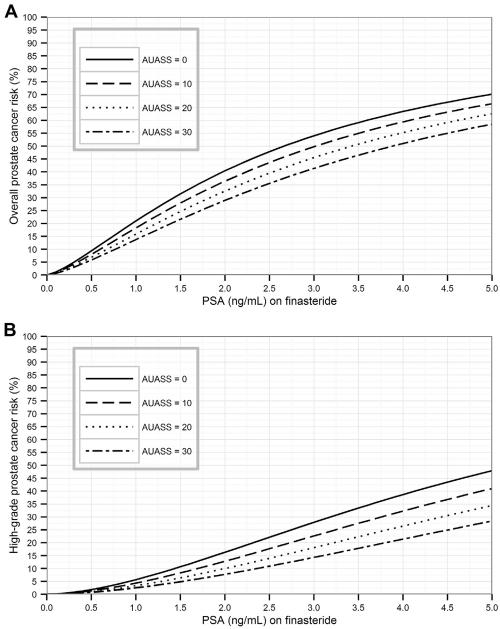
Risk curves in Figures 1 and 2 show the elevated risks of detection of prostate cancer and high-grade prostate cancer for low prostate volumes or low AUASS. Risk calculators for the 4 models are now available on the online PCPT Risk Calculator website along with analytical formulas to expedite external validation.
Figure 1.
(A) Overall and (B) high-grade prostate cancer risk curves incorporating prostate volume for a 65-year-old man with a normal digital rectal examination (DRE).
Figure 2.
(A) Overall and (B) high-grade prostate cancer risk incorporating American Urological Association symptom score (AUASS) for a 65-year-old man with no previous biopsy and a normal digital rectal examination (DRE).
COMMENT
Despite a significant fall in prostate cancer mortality since the inception of PSA screening, the use of PSA for early detection of prostate cancer has received enormous public attention because of a number of significant problems. These include the large number of negative (and therefore unnecessary) prostate biopsies and the detection of lower-grade prostate cancer, which often follows an indolent course for a man’s lifetime. These issues, in part, resulted in the United States Preventive Services Task Force’s recommendation against PSA testing in the general population.7
Major confounds related to PSA testing are because of the source of PSA and how biopsy is performed. Most PSA likely originates from benign enlargement (BPH) of the prostate, which increases with age, leading to false-positive “elevations” and unnecessary biopsies in many men. Regarding prostate biopsy, it is a relatively random process in which a biopsy needle is introduced into several regions of the prostate. In the initial years of the biopsy procedure, 4 cores were often obtained (1 each from upper-left, upper-right, lower-left, and lower-right portions of the prostate). For perhaps a decade or more, 6 cores were standard and more recently, 10-12 cores have become the norm. Both these confounds (PSA level and number of biopsy cores) are exacerbated by changes in prostate size that occur with age and interindividual differences: (1) men with larger prostates will generally have higher PSA values and even without prostate cancer, will be more likely to undergo biopsy, and (2) men with larger prostates will have a lower proportional sampling of the gland with a greater risk of not finding cancer.
Prompting our initial study in men in the placebo group of the PCPT was the hypothesis that the recent increase from 6 to 10-12 cores simply increased detection of low-grade, generally inconsequential cancer without increasing detection of high-grade, potentially lethal cancer. If this hypothesis had been confirmed, we would have recommended fewer prostate biopsies to reduce detection of inconsequential cancer and maintain detection of the larger, high-grade cancers, whereas reducing cost (fewer biopsies to process pathologically) and morbidity (presumably, fewer needle biopsies of a prostate would reduce the current 2%-4% risk of sepsis after biopsy). Our hypothesis did not prove to be correct: in the placebo group of the PCPT, we found that with increased numbers of biopsies, sensitivity for detection of high-grade cancer was increased. Confirming the importance of prostate volume in the general population, AUASS and prostate volume also had a significant effect on prostate cancer and high-grade cancer detection.5
In the present study, we asked the same question: are 10-12 biopsy cores necessary during prostate biopsy to optimally detect lethal, high-grade cancer in the man receiving finasteride? Surprisingly, the answer appears to be no. The likely explanation is that with the volume reduction in the prostate seen in these men because of the action of the medication, adequate sampling of the gland can be achieved with a smaller number of cores. Similarly, prostate volume and AUASS add little to risk assessment in men receiving finasteride.
The primary implication of this study is that for a man receiving finasteride in whom prostate cancer is suspected, a 10-12–core biopsy is not needed to adequately assess the risk of high-grade cancer; a 6-core biopsy will provide similar sensitivity for high-grade cancer detection. A reduction in the number of biopsy cores will reduce the cost of biopsy associated with pathologic interpretation, reduce patient discomfort and anxiety, and will likely reduce the risk of sepsis, a growing problem in the US.8 A secondary benefit arises from the observations related to volume and AUASS: our analysis demonstrates these to have only minor effect on risk assessment. As such, prebiopsy imaging for the determination of gland volume is unnecessary in the assessment of prostate cancer risk.
This analysis has a number of limitations. The decision for a participant in the PCPT to have more than 6 biopsy cores could be because of factors that were associated with risk of cancer, such as suspicion for prostate cancer. This would artificially induce an association between the number of cores and the cancer endpoints. Despite this likely bias, we found no statistically significant association. Second, the fraction of patients with >6 biopsy cores was small – 813 of 4509 biopsies (18.0%); nonetheless, it is a large number of patients, larger than many biomarker validation studies. There were greater proportions of missing volume and number of core values among interim compared with end-of-study biopsies, which contributed to more missing values among positive compared with negative biopsies (Table 1). The higher rate of missing biopsy information could be because more of the positive cases were identified from interim biopsies rather than required end-of-study biopsies, and local sites performing the biopsy were less likely to report details for the interim compared with end-of-study biopsies. Conclusions from this analysis are based on a population who had been taking finasteride for an extended duration of time, most as long as 7 years. It is not clear whether shorter-term prescriptions of finasteride would similarly imply that fewer cores suffice for cancer detection. Finally, the risk of up-grading and up-staging at radical prostatectomy is not negligible for this population, and this study has not addressed the potential effect of reduced cores on this risk.
Our findings are not in concurrence with those of Nguyen et al9 who found that the number of biopsy cores was related to prostate cancer risk in patients receiving dutasteride but not for risk of high-grade disease. However, their adjusted hazard ratio for number of cores indicates that a greater number of cores was associated with a reduced likelihood of detection for overall and high-grade prostate cancer on both the placebo and dutasteride arms. In addition, patients in their study were very different from the PCPT, as all their patients had undergone a previous negative prostate biopsy.
Because of the profound implications of our observations, especially related to the ability to perform fewer biopsies in men receiving finasteride, these observations merit serious consideration for replication in other populations to reduce cost and morbidity in this patient population.
CONCLUSION
For men receiving finasteride in the PCPT, the number of cores obtained at prostate biopsy did not affect the risk of prostate cancer detection, whereas prostate volume and AUASS had only a minor effect. In these men, physicians might consider a reduced number of biopsy cores, thereby reducing the cost and morbidity of prostate biopsy.
Acknowledgment
The authors gratefully acknowledge all PCPT participants.
Funding Support: This work was supported by grants U01CA86402, 5P30 CA054174-18, and (7)P01CA108964-06, and by Public Health Service grant CA37429 from the National Cancer Institute, Division of Cancer Prevention.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. New Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Pauler-Ankerst D, Chi C, et al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. JCO. 2007;25:3076–3081. doi: 10.1200/JCO.2006.07.6836. [DOI] [PubMed] [Google Scholar]
- 5.Ankerst DP, Till C, Boeck A, et al. The impact of prostate volume, number of biopsy cores, and AUA symptom score on the sensitivity of cancer detection using the Prostate Cancer Prevention Trial Risk Calculator. J Urol. 2013;190:70–76. doi: 10.1016/j.juro.2012.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucia MS, Epstein JI, Goodman PJ, et al. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. Ann InternMed. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 8.Pinkhasov GI, Lin YK, Palmerola R, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits – experience from 1000 consecutive cases. BJU Int. 2012;110:369–374. doi: 10.1111/j.1464-410X.2011.10926.x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen CT, Isariyawongse B, Yu C, Kattan MW. The REDUCE metagram: a comprehensive prediction tool for determining the utility of dutasteride chemoprevention in men at risk for prostate cancer. Front Oncol. 2012;2:138.1–138.7. doi: 10.3389/fonc.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]