Abstract
AIM
To investigate the acetylcholinesterase (AChE) expression involved in retina pigment epithelial (RPE) apoptosis induced by higher concentrations H2O2.
METHODS
The human retinal pigment epithelium cell line ARPE-19 was from ATCC (Rockville, MD). Cultured ARPE-19 cells were treated with H2O2 at 0, 250, 500, 1 000, 2 000µmol/L and cell viability was measured with MTT assay. AChE expression and DNA fragments were analyzed by immunocytochemistry, TUNEL and PARP-1 Western blotting.
RESULTS
Immunofluorescence detected AChE exist in the normal human retinal tissue. When H2O2 >500µmol/L, AChE expression showed an increase after 2h, and this concentration was selected for the present study. RPE cell was induced with 1 000µmol/L H2O2 for 2h, compared to the control group, cell activity decline detected by MTT, AChE and PARP-1 protein expression was significantly increased detected by Western blotting. AChE immunofluorescence staining was positive in RPE cell after H2O2 incubate 2h. In addition, pretreatment with 100µmol/L epigallocatechin gallate (EGCG), cell viability increased from 31.20%±3.90% to 70.23%±12.96%.
CONCLUSION
AChE is weakly expressed in normal human RPE cells. Stimulation with H2O2 caused the stable increase of AChE expression in RPE cells, which may indicate that AChE may be an important role in AMD.
Keywords: acetylcholinesterase, retina pigment epithelial cells, oxidative stress, age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD) is the most common cause of severe loss of vision in developed countries[1]-[3]. It is characterized by the progressive loss of central vision and the degeneration of retinal photoreceptors, retina pigment epithelial (RPE) and Bruch's membrane. The pathogenesis of AMD, which covers a complex interaction of genetic and environmental factors, is strongly associated with chronic oxidative stress that ultimately leads to protein damage and degeneration of RPE. Specific findings for AMD include accumulation of intracellular lipofuscin and extracellular drusen[4]-[6]. High oxygen tension, exposure to light, and biochemical events of vision generate significant oxidative stress in RPE, followed with the degeneration of retinal photoreceptors and RPE cells[6].
The susceptibility of neural retina and RPE cells to oxidative damage appears to be a major factor in retinal degeneration. Oxidative injury to RPE has been thought to play a key role in AMD[7]. New evidences suggest that oxidative stress may be a potential inducer of inflammation response in human RPE cells. The RPE monolayer is at the risk of oxidative damage owing to its exposure to high levels of visible light and oxygen in normal conditions. At high concentrations, H2O2 induces ARPE-19 cell death through a regulated necrotic pathway with calcium overload as a critical step in the cell death program[10]. We have also reported that acetylcholinesterase (AChE) expression is up-regulated during the apoptosis of cells that originate from non-muscle, non-nervous or non-hematopoietic systems, which suggests that AChE might be a novel regulator of apoptosis. In 1972, Kerr et al[12] used a Greek term ‘apoptosis’ to describe the morphological depiction of cell death. The loss of vision in retinal degeneration disease associates with oxidative stress and apoptosis in RPE cell[13]. Oxidative stress in the RPE is hypothesized to be a major contributor to the development of AMD[14].
AChE is encoded by a single gene-ACHE, while three different isoforms exist: synaptic (S) or tail (T), erythrocytic (E) and read-through (R)[15]. However, it is becoming clear that AChE has a range of actions. Its functions have been identified in apoptosis, stress-responses, neuritogenesis, and neurodegeneration. Furthermore, these non-classical roles are attributable not only to the native protein, which acts as a mediary binding protein under a number of circumstances, but also to peptides cleaved from AChE can act as independent signaling molecules[16]. AChE is notably involved in neuronal stress reactions[17],[18]. The effects of AChE depend on the cell type and cell-differentiation state, the modulation of expression levels, cellular distribution and binding with its protein partners[19]. Our researches have demonstrated that AChE is an important contributor to the induction of apoptosis in various cell types[20]. Oxidative stress plays an important role in RPE death during aging and the development of age-related macular degeneration. Although early reports indicate that reactive oxygen species (ROS) including H2O2 can trigger apoptosis at lower concentrations and necrosis at higher concentrations, the exact molecular mechanism of AChE involved in RPE apoptosis is still unclear. We provide evidence to suggest that the induction of AChE expression during apoptosis is regulated by the mobilization of intracellular Ca2+ [9]. We detected that AChE exist in the normal human retina, during apoptosis, a significant increase in AChE protein level strongly suggest that AChE play a key role during the apoptosis of RPE cells.
MATERIALS AND METHODS
Materials
Hydrogen peroxide were purchased from Shanghai Shanghai Academy Biotechnology Research Center (CASB) Biotechnology. The rabbit polyclonal anti-cleaved caspase-3 antibody (9661) were purchased from Cell Signaling Technology (Beverly, MA, USA). The AChE monoclonal antibody detected endogenous levels of a 68kDa fragment of human AChE was from BD Biosciences (San Jose, CA, USA). Rhodamine coupled anti-mouse IgG and fluorescein isothiocyanate (FITC) coupled anti-rabbit IgG were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cell pellets were suspended in TUNEL reaction mixture (Roche Diagnostics Corporation Indianapolis, IN, USA) for 1h and stained with Hoechst33258. Stained cells were analyzed with a Nikon fluorescence microscope (Nikon Inc.). epigallocatechin gallate (EGCG) were purchased from Sigma (St Louis, USA).
Methods
Human eye tissue
All studies using human tissues were in accordance with the tenets of the Declaration of Helsinki and in accordance with the policies of the institutional review board for human subjects, and were approved by the Ethics Committee of Eye Hospital Affiliated Nanchang University, Normal human eye tissue were obtained from the Cornea and Ocular Surface Clinic of Eye Hospital Affiliated Nanchang University.
Oxidative stress of retina pigment epithelial cells
The hRPE cell line, ARPE-19 (ATCC, Manassas, VA, USA), were cultured in 1:1 DMEM/F12 with 10% fetal bovine serum, 100U/mL penicillin, and 100mg/mL streptomycin. Cells were grown at 37°C in a humidified atmosphere of 95% air 5% CO2. The cells were passed every 3-4d by digestion with 0.05% trypsin/0.02% ethylenediaminetetraacetic acid (EDTA). 10×105 cells per 10cm dish were seeded for 24h. A stock concentration of 3% hydrogen peroxide (H2O2, 0.88mol/L) was diluted in the growth media to attain final concentrations ranging from 0.2 to 10mmol/L. The treated cells were incubated for 24h. The cytotoxicity was assayed by mitochondrial function using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) testing. by phase contrast microscopy and stained with AChE, Hoechst 33258 and TUNEL.
Retina pigment epithelial cells apoptosis
RPE Cells were prepared as described, which were homogenized and solubilized in ice cold PBS containing protease inhibitors, phenylmethylsulfonyl fluoride (1mg/L), aprotinin (1mg/L), leupeptin (1mg/L), pepstatin A (1mg/L) and EDTA (1mmol/L). The homogenate was centrifuged at 15 000r/min at 4°C for 10min. The protein content of the supernatants was determined by the Bradford method. After sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12% linear slab gel, under reducing conditions, separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. Blot was stained at room temperature with a 1:100 dilution of monoclonal mouse anti-AChE antibody (1:400) over night at 4°C. After washing and incubation with horseradish peroxidase-conjugated secondary antibody (1:5 000 dilution), blot was developed using the enhanced chemiluminescence and analyzed with Western blot analysis detection system.
Western blot analysis
The RPE cells were homogenized by 200µL of lysis-buffer (20mmol/L Tris, pH 7.4, 150mmol/L NaCl, 1mmol/LEDTA, 1mmol/L orthovanadate, 1mmol/L phenylmethylsulfonyl fluoride, 1µg/mL leupeptin and 10µg/mL aprotinin) on ice. The protein concentration of the sample was determined using the Bradford assay. Equal amounts (50µg) of proteins from each sample were loaded on SDS-PAGE and then transferred onto PVDF membrane (Millipore, USA) at 100mA for 2h. After blocking of nonspecific binding sites with 5% skim milk for 1h, the membrane was incubated with the primary antibodies as the following dilutions: mouse monoclonal antibody to AChE at 1:200 (Santa Cruz, CA), and rabbit polyclonal antibodies to PARP-1at 1:200 overnight at 4°C. Antibody dilutions were made in a solution of 5% skim milk/0.1% Tris-buffered saline Tween-20. Then, membranes were washed with TBS and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Bioss Biotechnology, Beijing, China) in blocking buffer for 1h at room temperature. After four washes, the proteins were detected with ECL kit (thermo fisher, US). β-actin staining served as the internal standard for all membranes.
Double staining of AChE and TUNEL
To ensure that the AChE protein indeed exists in apoptotic cells, we performed double staining with immunocytochemistry for AChE and TUNEL reaction for DNA breaks. Briefly, after 20min fixation at 4°C with paraformaldehyde solution (3% in PBS, pH 7.4), harvested cells were washed three times with TBST [50mmol/L Tris-HCl (pH 7.4), 150mmol/L NaCl, 0.1% Tween]. The cells were incubated with 1mL of Blocking buffer (5.5% Normal Goat Serum in TBST) for 45min and then incubated with 100µL primary antibody (1:100 dilution in TBST containing 2% BSA) for 24h at 4°C. Following incubation, the cells were washed and incubated with 100µL of secondary antibody (1:100 dilution, rhodamine conjugated anti-mouse IgG-R, Santa Cruz, CA, USA) for 60min at 37°C in the dark. The cells were then washed and re-suspended in a permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2min on ice. After washing, the pellet was re-suspended in 50µL TUNEL reaction mixture (Roche) and incubated for 60min at 37°C in the dark. The labeled cells were then washed, transferred onto glass slides, and observed under a fluorescence microscope (OLYMPUS).
Statistical Analysis
All experiments were repeated at least three times, Data are expressed as the mean±SEM of four replicate samples. The results were analyzed by Student's unpaired t-test, to determine the significant difference between means, or by two-way ANOVA followed by a least-significance procedure, to determine the significance of the response. P<0.05 was considered significant.
RESULTS
AChE Exists in Normal Retinal Tissue
Human (Eye Hospital Affiliated Nanchang University donors) was fixed and made the paraffin sections (5µm). 4′,6-diamidino-2-phenylindole (DAPI) and AChE immunofluorescence staining (Figure 1), AChE immunofluorescence is green, and AChE exists in the cytoplasm of the RPE cells, DAPI specificity in the nucleus.
Figure 1. HE and AChE immunofluorescence staining in normal human retina tissue.
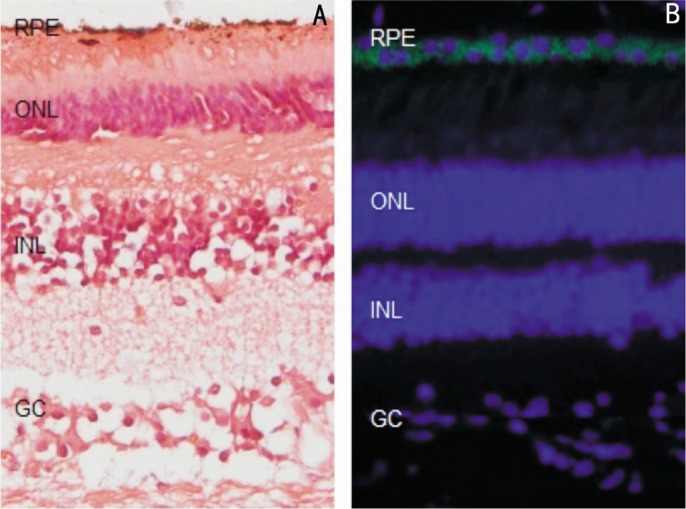
HE staining of human retina (A), AChE immunofluorescence is green, DAPI specificity in the nucleus (blue) is merged (400×).
In Vitro ARPE-19 Cells Lines Biological Characteristics
An inverted phase contrast morphological changes of human RPE cells under the microscope before and after the injury induced by H2O2. Normal human RPE cells are the main cell body long spindle-shaped or triangular opaque cells blurring. After being induced with 250µmol/L H2O2 for 2h, no cellular morphology change was observed. However, after being incubated with 500µmol/L H2O2 for 2h, with the elevated concentrations of H2O2, there is cell fragmentation, floating and death (Figure 2A). The visible cell number reduced compare to control group (Figure 2B).
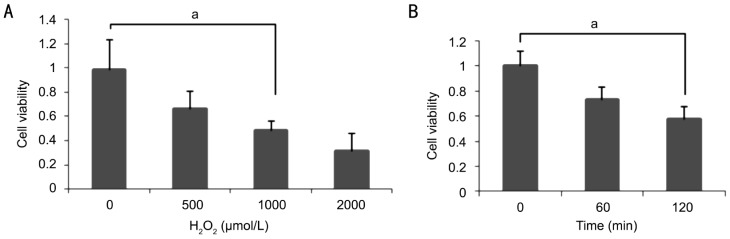
Figure 2. The time and concentration dose of RPE cell treated by H2O2.
Detect the RPE cells viability via MTT assay and the result shows that: treated concentration are shown at A (0, 500, 1 000, 2 000µmol/L), with the increase concentration of H2O2, the cell survive rate decrease, as well as treated time at B (0, 60, 120min) when the H2O2 concentration is 1 000µmol/L, 120min has the high rate about 50%, aP<0.05.
MTT Assay of H2O2 on the Growth Of Human Retina Pigment Epithelial Cells
1 000µmol/L H2O2 for 2h can significantly inhibit the growth of ARPE-19 Cell, A value of 490nm wavelength filter measured calculated inhibition rates, namely: cell inhibition rate=(A value of the control group-experimental group A value)/A value of the control group×100%, compared with the control group, 250µmol/L 500µmol/L 1 000µmol/L 2 000µmol/L is lower, the difference was statistically significant (Figure 3), P<0.05.
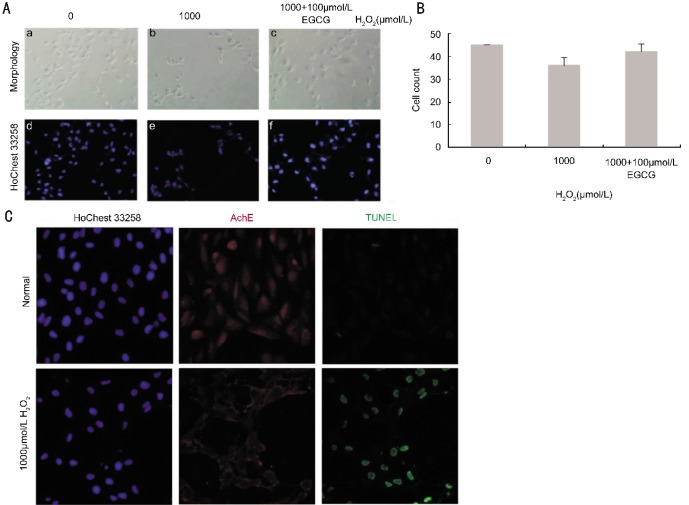
Figure 3. Induction apoptosis of RPE cells treated with H2O2 by morphology and TUNEL.
Control group (a,d), after 2h following H2O2 (1 000µmol/L), these is cell fragmentation, floating and death (b,e), 2h following H2O2 (1 000µmol/L) and 100µmol/L EGCG (c, f) pre-treatment, cell number is more (A). The HoChest 33258 staining is positive, AChE immunofluorescence and TUNEL staining is negative in control group. HoChest 33258, AChE immunofluorescence and TUNEL all positive staining in H2O2 (1 000µmol/L) group 400×(C).
AChE Expression in H2O2-Triggered Apoptotic Retina Pigment Epithelial Cells
H2O2 (1 000µmol/L) treatment can induce apoptosis of RPE cells by increasing ROS levels[21]. We treated RPE cells with H2O2 (1 000µmol/L) for 2h, Leika confocal microscope photograph shows the normal growth of RPE cells HoChest 33258 staining positive, AChE immunofluorescence and TUNEL staining negative. However, TUNEL and AChE immunofluorescence staining in H2O2 group is positive (Figure 3). These data suggested that the expression of AChE was induced in apoptotic cells, means that H2O2 (1 000µmol/L) treated for 2h can induce RPE cell apoptosis with the AChE expression, proved that AChE may play a key role in the apoptosis of human RPE cells.
H2O2 Induces AChE Expression in Human RPE Cells
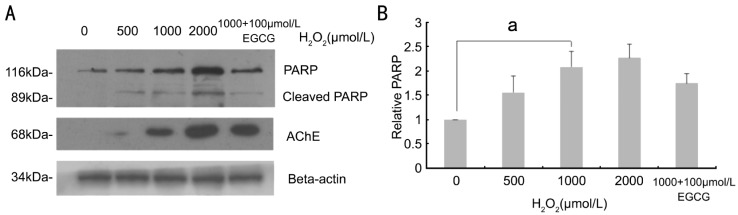
Western blotting results showed a weak AChE bands in normal control group, indicating that AChE protein was slightly expressed in normal human RPE cells. After 2h, the expression of AChE was slightly enhanced in 500µmol/L H2O2 group and significantly enhanced in 1 000µmol/L H2O2 group. The cleavage of PARP was found starting at 2h after treatment (Figure 4A). Calculate the relative integral value of A, as the relative expression level of AChE protein. The statistical analysis of the results showed that the relative integral. A comparison of AChE expression in 500µmol/L H2O2 group, 1 000µmol/L group and control group, AChE relative expression increased significantly, the differences were statistically significant (Figure 4B).
Figure 4. After different concentrations of H2O2 incubated for 2h, the AChE protein is detected by western blot (A).
With the higher concentration of H2O2, cleaved-PARP, AChE and beta-actin Western blot, cleaved-PARP, AChE protein show an increase trend. (B) Western blot gray value quantitative is analyzed (ImageJ software), aP<0.05, statistically significant.
DISCUSSION
It was demonstrated that the apoptosis of ARPE-19 cells induced with low concentration of H2O2 was related to the activation of caspase-3, while the apoptosis of ARPE-19 cells at high concentration of H2O2 was mediated by calcium overload[10],[22]. We observed that RPE cells were tolerant of low concentration H2O2 in a short time; nevertheless, prolonged treatment increased the death of RPE cells. On the other hand, we found that H2O2 would cause a massive cell apoptosis at concentrations over 500µmol/L for 2h. Moreover, we confirmed that necrosis of ARPE-19 cells induced with high concentrations H2O2 was a regular process. We supported our standpoint by pretreatment of ARPE-19 cells with antioxidant EGCG to attenuate the H2O2-induced injury. Therefore, H2O2 may induce the destruction of RPE cells in AMD by the combined effects of apoptosis and necrosis.
It has been reported that AChE is slightly expressed in normal RPE cells, however, our study firstly showed that the AChE expression was upregulated in the H2O2-induced apoptosis of RPE cells, furthermore,with the increase of H2O2 concentration, AChE expression varied accordingly, However, the exact apoptotic pathways involved in the AChE-mediated apoptosis remain to be further elucidated.
High concentration of H2O2 affects the expression, as well as the function of intracellular enzymes[29]-[31]. After being treated with 1 000µmol/L H2O2 for 2h, RPE cells were performed with TUNEL staining. The results indicated that H2O2 caused the apoptosis of RPE cells. At the same time, AChE and PARP-1(poly ADP-ribose polymerase) were significantly up-regulated, which indicated that AChE was involved in apoptosis of ARPE-19 cells. PARP, which is activated by oxidative stress generated from DNA strand braid break, plays an important role in protecting RPE cells from high oxygen tension[32]. From what is mentioned above we can reach the conclusion that AChE involved in the process of ARPE-19 cell necrosis. However, more investigations are requested to clarify the AChE regulatory mechanisms involved in apoptosis of RPE cells.
An increasing number of studies have shown that the occurrence of AMD correlates with the functional impairment of RPE cells[6]. We have confirmed that AChE is slightly expressed in normal human retina, and the expression level is up-regulated during the apoptosis of RPE cells. Therefore, we can reach a conclusion that AChE may be an important factor in the genesis of AMD.
AChE inhibitors such as Tacrine, Donepezil have been widely used in the clinical treatment of Alzheimer's disease (AD). The mechanism is to increase the amount of ACh at receptor sites. Besides, there seems to be other mechanisms for donepezil to take effect, including the interaction with some specific peptides, neurotransmitter receptors, and Ca2+ channels. High concentration H2O2 mainly induced the calcium overload and the consequent necrosis of RPE cells. From this we learn that AChE inhibitors may take effect via Ca2+ channels to suppress the apoptosis of RPE cells and then to slow down the process of AMD, which provides us with a new therapeutic target for AMD and a new research aspect of cell protection.
Acknowledgments
We thank Eye Hospital Affiliated Nanchang University for providing normal human retina tissue.
Footnotes
Foundation items: National Natural Science Foundation of China (No.31071213, 81101479, 30971481, 81260148, 81271425 and 81160118); Clinical Medicine Research Special-purpose Foundation of China (No.L2012052); Natural Science Foundation of Jiangxi Province, China (No.20114BAB215029); Technology Foundation of Jiangxi Province, China (No 20111BBG70026-2); Health Department Science and Technology Foundation of Jiangxi Province, China (No.20121026); Education Department Youth Scientific Research Foundation of Jiangxi Province, China (No.GJJ12158)
REFERENCES
- 1.Cheung CM, Tai ES, Kawasaki R, Tay WT, Lee JL, Hamzah H, Wong TY. Prevalence of and risk factors for age-related macular degeneration in a multiethnic Asian cohort. Arch Ophthalmol. 2012;130(4):480–486. doi: 10.1001/archophthalmol.2011.376. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 3.Minassian DC, Reidy A, Lightstone A, Desai P. Modelling the prevalence of age-related macular degeneration (2010-2020) in the UK: expected impact of anti-vascular endothelial growth factor (VEGF) therapy. Br J Ophthalmol. 2011;95(10):1433–1436. doi: 10.1136/bjo.2010.195370. [DOI] [PubMed] [Google Scholar]
- 4.Cai X, McGinnis JF. Oxidative stress: the achilles' heel of neurodegenerative diseases of the retina. Front Biosci. 2012;17:1976–1995. doi: 10.2741/4033. [DOI] [PubMed] [Google Scholar]
- 5.Cano M, Thimmalappula R, Fujihara M, Nagai N, Sporn M, Wang AL, Neufeld AH, Biswal S, Handa JT. Cigarette smoking, oxidative stress, the anti-oxidant response through Nrf2 signaling, and Age-related Macular Degeneration. Vision Res. 2010;50(7):652–664. doi: 10.1016/j.visres.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90(4):299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 7.Cao G, Chen M, Song Q, Liu Y, Xie L, Han Y, Liu Z, Ji Y, Jiang Q. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells. Mol Med Rep. 2012;5(1):54–59. doi: 10.3892/mmr.2011.582. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Gao W, Jiang H, Jin QH, Shi YF, Tsim KW, Zhang XJ. Regulation of acetylcholinesterase expression by calcium signaling during calcium ionophore A23187- and thapsigargin-induced apoptosis. Int J Biochem Cell Biol. 2007;39(1):93–108. doi: 10.1016/j.biocel.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674(1–2):36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Li GY, Fan B, Zheng YC. Calcium overload is a critical step in programmed necrosis of ARPE-19 cells induced by high-concentration HO. Biomed Environ Sci. 2010;23(5):371–377. doi: 10.1016/S0895-3988(10)60078-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Yang L, Yu L, Lin B, Hou Y, Wu J, Huang Q, Han Y, Guo L, Ouyang Q, Zhang B, Lu L, Zhang X. Acetylcholinesterase is associated with apoptosis in beta cells and contributes to insulin-dependent diabetes mellitus pathogenesis. Acta Biochim Biophys Sin (Shanghai) 2012;44(3):207–216. doi: 10.1093/abbs/gmr121. [DOI] [PubMed] [Google Scholar]
- 12.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, Keyes KT, Long B, Chen G, Ye Y. Impact of HMG-CoA reductase inhibition on oxidant-induced injury in human retinal pigment epithelium cells. J Cell Biochem. 2011;112(9):2480–2489. doi: 10.1002/jcb.23173. [DOI] [PubMed] [Google Scholar]
- 14.Seo SJ, Krebs MP, Mao H, Jones K, Conners M, Lewin AS. Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp Eye Res. 2012;101:60–71. doi: 10.1016/j.exer.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soreq H, Seidman S. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2001;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 16.Halliday AC, Greenfield SA. From protein to peptides: a spectrum of non-hydrolytic functions of acetylcholinesterase. Protein Pept Lett. 2012;19(2):165–172. doi: 10.2174/092986612799080149. [DOI] [PubMed] [Google Scholar]
- 17.Meshorer E, Soreq H. Virtues and woes of AChE alternative splicing in stress-related neuropathologies. Trends Neurosci. 2006;29(4):216–224. doi: 10.1016/j.tins.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.López-Granero C, Cañadas F, Cardona D, Yu Y, Giménez E, Lozano R, Avila DS, Aschner M, Sánchez-Santed F. Chlorpyrifos-, Diisopropylphosphorofluoridate- and Parathion-induced behavioral and oxidative stress effects: Are they mediated by analogous mechanisms of action? Toxicol Sci. 2013;131(1):206–216. doi: 10.1093/toxsci/kfs280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H, Zhang XJ. Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. FEBS J. 2008;275(4):612–617. doi: 10.1111/j.1742-4658.2007.06236.x. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Jiang H, Wan YH, Du AY, Guo KJ, Liu T, Ye WY, Niu X, Wu J, Dong XQ, Zhang XJ. Induction of a 55 kDa acetylcholinesterase protein during apoptosis and its negative regulation by the Akt pathway. J Mol Cell Biol. 2011;3(4):250–259. doi: 10.1093/jmcb/mjq047. [DOI] [PubMed] [Google Scholar]
- 21.Kannan R, Jin M, Gamulescu MA, Hinton DR. Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Radic Biol Med. 2004;37(2):166–175. doi: 10.1016/j.freeradbiomed.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Bazan NG. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Adv Exp Med Biol. 2006;572:531–540. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]
- 23.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18(54):7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 24.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22(15):3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventura JJ, Cogswell P, Flavell RA, Baldwin AS, Jr, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18(23):2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Festjens N, Kalai M, Smet J, Meeus A, Van Coster R, Saelens X, Vandenabeele P. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ. 2006;13(1):166–169. doi: 10.1038/sj.cdd.4401746. [DOI] [PubMed] [Google Scholar]
- 27.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. A Apoptosis. 2007;12(5):913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 28.Morgan MJ, Kim YS, Liu Z. Lipid rafts and oxidative stress-induced cell death. A Antioxid Redox Signal. 2007;9(9):1471–1483. doi: 10.1089/ars.2007.1658. [DOI] [PubMed] [Google Scholar]
- 29.Bai L, Yan HH, Zhang DX, Wang JM, Sun NX. Effects of oxidative stress on barrier function of human retina pigment epithelium and its molecular mechanisms. Zhonghua yanke zazhi. 2012;48(5):417–422. [PubMed] [Google Scholar]
- 30.Kim MH, Chung J, Yang JW, Chung SM, Kwag NH, Yoo JS. Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, ARPE-19. Korean J Ophthalmol. 2003;17(1):19–28. doi: 10.3341/kjo.2003.17.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Xu GX, Xiao ZY, Xie MS, Feng YL, Guo J, Fu LX. Protective effects of melatonin on cultural human retinal pigment epithelial cells against oxidative damage in vitro. Zhonghua yanke zazhi. 2009;45(6):528–532. [PubMed] [Google Scholar]
- 32.Jarrett SG, Boulton ME. Poly(ADP-ribose) polymerase offers protection against oxidative and alkylation damage to the nuclear and mitochondrial genomes of the retinal pigment epithelium. Ophthalmic Res. 2007;39(4):213–223. doi: 10.1159/000104683. [DOI] [PubMed] [Google Scholar]