Figure 3.
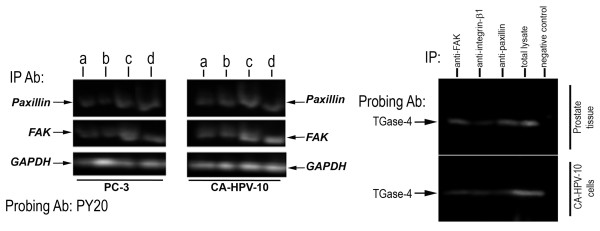
TGase-4 and the activation of the focal adhesion complex. LEFT: effect of exogenous TGase-4 on the activation of focal adhesion proteins. Cells were first subject to serum hunger for 2 hours, before treated with: a-serum free medium; b-serum free medium with BSS/BSA buffer; c-rhTGase-4 100 ng/ml and d-sodiium orthovanadate (with 0.1% hudrogen peroxide). After one hour, cells were pelleted and lysed. Anti-FAK or anti-paxillin antibody was added to the protein lysate. Immunoprecipiate was separated on 8% SDS-PAGE gel and probed by PY20 antibody. Total cell lysate used for immunoprecipitation were probed by anti-GAPDH antibody as a load control. rhTGase-4 induced phosphorylation of FAK particular in PC-3 cells, and also induced phosphorylation of paxillin. RIGHT: Interaction between TGase-4 and focal adhesion complex proteins. Proteins from fresh frozen human prostate tissues (top) and from a TGase-4 positive CA-HPV-10 cells (bottom) were precipitated with anti-FAK, anti-integrinb1, anti-paxillin or irrelevant antibody (negative control). The immunoprecipitates or total protein lysate from prostate tissues (total lysate, used as a positive control) were separated by SDS-PAGE. TGase-4 was probed with an anti-TGase-4 monoclonal antibody (MaxPaB, Abnova). FAK and paxillin were strongly associated with TGase-4, compared with integrin.
