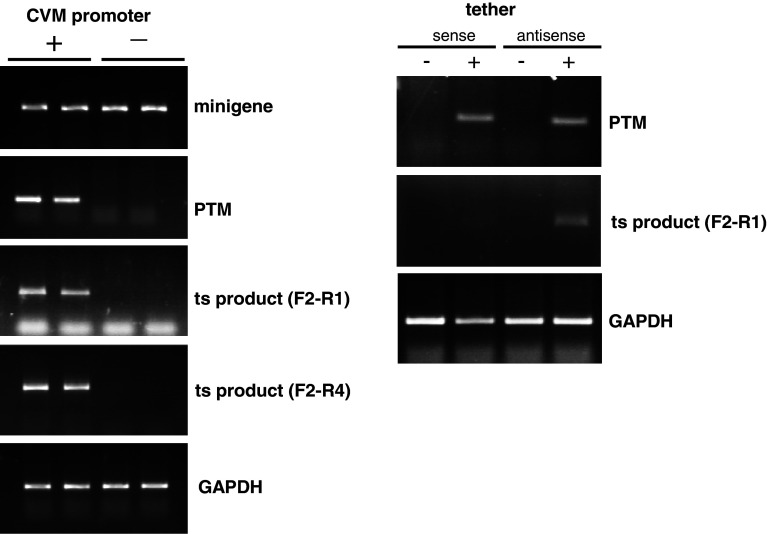
Fig. 4.
The trans-splicing product is generated by interaction of RNA molecules, not via plasmid DNA recombination. a HEK293 cells were co-transfected with identical amounts of the minigene plasmid and a PTM construct either with (left lanes, labeled “+”) or without (right lanes, labeled “−”) the CMV promoter. After RNA isolation and RT-PCR using appropriate primer pairs (Fig. 3), mRNA from the minigene (top panel) or the PTM (second panel) was detected. As expected, minigene expression levels were comparable with or without the CMV promoter in the PTM construct. However, in the absence of the CMV promoter in the PTM, only a very small amount of PTM mRNA was detected. Residual levels of PTM RNA which can be detected after prolonged PCR (data not shown) are likely due to the presence of AAV ITRs in the PTM plasmid which have weak intrinsic promoter activity. However, no trans-splicing product was detected using reverse primers for either exon 2 (third panel, F2–R1) or exon 3 (fourth panel, F2–R4) when the CMV promoter was absent in the PTM. GAPDH was used as internal control. Duplicate reactions are shown. b A PTM plasmid in which the 100-bp tether domain sequence (antisense) was replaced by its reverse complement (sense) was co-transfected with the minigene construct into HEK293 cells. With this sense construct, the trans-splicing reaction was much less effective