Abstract
Purpose
To identify prognostic factors for the outcomes of empirical antifungal therapy, we performed a multicenter, prospective, observational study in immunocompromised patients with hematological malignancies.
Materials and Methods
Three hundred seventy-six patients (median age of 48) who had neutropenic fever and who received intravenous (IV) itraconazole as an empirical antifungal therapy for 3 or more days were analyzed. The patients with possible or probable categories of invasive fungal disease (IFD) were enrolled.
Results
The overall success rate was 51.3% (196/376). Age >50 years, underlying lung disease (co-morbidity), poor performance status [Eastern Cooperative Oncology Group (ECOG) ≥2], radiologic evidence of IFD, longer duration of baseline neutropenic fever (≥4 days), no antifungal prophylaxis or prophylactic use of antifungal agents other than itraconazole, and high tumor burden were associated with decreased success rate in univariate analysis. In multivariate analysis, age >50 years (p=0.009) and poor ECOG performance status (p=0.005) were significantly associated with poor outcomes of empirical antifungal therapy. Twenty-two patients (5.9%) discontinued itraconazole therapy due to toxicity.
Conclusion
We concluded that empirical antifungal therapy with IV itraconazole in immunocompromised patients is effective and safe. Additionally, age over 50 years and poor performance status were poor prognostic factors for the outcomes of empirical antifungal therapy with IV itraconazole.
Keywords: Hematological malignancy, prognosis, itraconazole, empirical antifungal therapy
INTRODUCTION
Invasive fungal disease (IFD) is an important cause of morbidity and mortality in patients with hematological malignancies who receive intensive chemotherapy or hematopoietic stem cell transplantation (HSCT), and the incidence of IFD has increased over the past decades.1-3 Recently, the incidence of Candida infections has decreased due to the widespread prophylactic use of antifungal agents such as fluconazole, whereas Aspergillus infections have become the most common cause of IFD.2,3 Delayed diagnosis and treatment of invasive Aspergillus infections usually lead to fatal clinical outcomes associated with IFD. However, invasive Aspergillus infections are often difficult to diagnose due to nonspecific clinical presentations. Moreover, the identification of Aspergillus species from sterile specimens by invasive procedures may be impractical in patients with unstable clinical situations or with a bleeding tendency. Therefore, empirical antifungal therapy for suspected IFD was established for febrile neutropenic patients with hematological malignancies who present with persistent or recurrent unexplained fever despite the use of broad-spectrum antibiotics.4,5
Although empirical antifungal therapy with deoxycholate amphotericin B (D-AMB) has reduced the incidence of breakthrough IFD, several serious toxicities related with D-AMB, including nephrotoxicity, have been frequently reported.6,7 Therefore, liposomal amphotericin B (L-AMB), mold active azoles (itraconazole, voriconazole and posaconazole) or caspofungin are usually recommended instead of D-AMB for empirical antifungal therapy.8-10 Because these antifungal agents for empirical antifungal therapy have similar efficacy and safety,4,8,9,11 the main concern of empirical antifungal therapy is no longer choosing the best agent, but rather the identification of patients who will be most likely to benefit from a given antifungal agent. Several years ago, intravenous (IV) itraconazole became available in Korea, and a relatively low incidence of toxicity has been reported.6,7
Although there have been many reports on the efficacy and safety of IV itraconazole for empirical antifungal therapy,6,7,12,13 there have been few reports on the prognostic factors for predicting the outcomes of empirical antifungal therapy. In this prospective, multicenter, observational study, we assessed the success rate of empirical antifungal therapy with IV itraconazole in immunocompromised patients with hematological malignancies. Additionally, we attempted to evaluate prognostic factors for predicting the outcomes of empirical IV itraconazole therapy among clinical data.
MATERIALS AND METHODS
Study design
This prospective multicenter observational study was performed at 26 medical centers in Korea during the period of April 2007 to December 2007. During the period of enrollment, 390 patients with hematological malignancies, such as acute leukemia, malignant lymphoma, myelodysplastic syndrome (MDS) or multiple myeloma (MM), who had neutropenic fever were enrolled. The primary objective of this study was to evaluate the overall success rate of empirical antifungal therapy with IV itraconazole, and the secondary objective was to define the poor prognostic factors for the outcomes of empirical antifungal therapy with IV itraconazole. This protocol was reviewed and approved by the ethics committee or institutional review board of each participating institution. Written informed consent was obtained from all participants before enrollment. This study was conducted according to the Declaration of Helsinki. We used a web-based electronic case report form to gather demographic information and various clinical characteristics of the participants. Central monitors conducted both central online data monitoring and on-site monitoring visits to improve data quality.
Patients aged 18 years or older with persistent (more than 2 days) or recurrent neutropenic fever,14 despite empirical broad-spectrum systemic antibacterial therapy, were eligible to be enrolled in this study. Fever was defined as a single oral temperature above 38.3℃ or a temperature above 38.0℃ for 1 hour.10,14 Neutropenia was defined as an absolute neutrophil count (ANC) below 0.5×109/L or an ANC below 1.0×109/L with a predicted decrease to below 0.5×109/L.10,14 The patients with possible or probable categories of IFD according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) consensus criteria were able to be enrolled in this study.15,16 Patients were not eligible if they had a documented (proven) IFD according to the EORTC/MSG consensus criteria, except Candida infections including candidemia. Other exclusion criteria were a history of serious allergy to itraconazole, a bilirubin level more than three times the upper limit of normal (ULN), an aminotransferase or alkaline phosphatase level more than five times the ULN, and severe renal dysfunction (a calculated creatinine clearance below 30 mL/min). We also excluded pregnant women and patients who were treated with combination therapy with other antifungal agents.
Patients were divided into high- or low-risk groups based on underlying tumor burden. High-risk patients included those who had newly diagnosed acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), MDS with a higher score according to the International Prognostic Scoring System (IPSS intermediate-2 or more),17 greater than low-intermediate risk of non-Hodgkin's lymphoma (NHL) according to the age-adjusted International Prognostic Index (aaIPI≥2),18 advanced-stage Hodgkin lymphoma (HL) according to the Ann Arbor staging system (≥stage 3),19 and MM with a high International Staging System score (stage 3).20 Relapsed or refractory patients were also considered high-risk in this study. Among the patients who had undergone HSCT, the high-risk group was defined as acute leukemia not in first complete remission (CR), malignant lymphoma or MM not in CR, and advanced-stage MDS (IPSS intermediate-2 or more).21
Treatment
IV itraconazole (Janssen Pharmaceutica, Beerse, Belgium) treatment was initiated at 200 mg every 12 hours for the first 48 hours, and followed by 200 mg once a day from day 3 to 14. After using IV itraconazole, an itraconazole oral solution (Janssen Pharmaceutica) at a dose of 200-400 mg/day replaced IV itraconazole when necessary. Participants continued treatment with itraconazole until defervescence and recovery from neutropenia (ANC over 500 cells/uL for at least 3 successive days). Each investigator could determine the duration of empirical antifungal therapy for the patients with baseline or breakthrough fungal infections. However, it was recommended that itraconazole treatment should be given for at least 14 days or for at least 7 days after resolution of neutropenia and symptoms. Efficacy and safety analyses were conducted for the patients who received IV itraconazole for 3 or more days. Patients were observed for up to 7 days after completion of treatment with itraconazole for clinical or microbiological evidence of fungal infection.
Analysis of efficacy and safety
The assessments of efficacy for empirical antifungal therapy were performed according to the previously reported five endpoints.7,22-24 We considered the outcomes of empirical antifungal therapy as successful if all five of the following criteria were met: 1) successful treatment of baseline fungal infection, 2) absence of any breakthrough fungal infection during empirical antifungal therapy and within 7 days after the completion of empirical antifungal therapy, 3) survival for at least 7 days after discontinuation of empirical antifungal therapy, 4) resolution of fever (defined as a temperature below 38℃ for at least 2 days) prior to recovery from neutropenia, and 5) no premature discontinuation of empirical antifungal therapy due to drug-related toxicity or lack of efficacy.
Baseline fungal infections were defined as those that developed within the first 48 hours after starting empirical antifungal therapy. Breakthrough fungal infections were defined as those that occurred after 3 days of empirical antifungal therapy or within 7 days after completion of empirical antifungal therapy. The diagnosis of breakthrough fungal infection was confirmed when the patients were diagnosed as proven or probable IFD according to the EORTC/MSG consensus criteria.16
The safety assessments for empirical antifungal therapy with itraconazole were performed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Effects, version 3.0.25 Clinical adverse events were monitored prospectively during the study period. Toxicities of itraconazole, including hepatotoxicity and nephrotoxicity, were defined when the grade of toxicities reached grade 2 or more and an increment of one or more grades above the baseline grade level.
Statistical analysis
The chi-square test was used to compare the success rate of empirical antifungal therapy between the high-risk group and low-risk group. We also performed the chi-square test for evaluating effects of baseline clinical characteristics on the outcomes of empirical antifungal therapy with IV itraconazole. Stepwise multivariable logistic regression was used to identify poor prognostic factors associated with a poor outcome of empirical antifungal therapy with IV itraconazole. We did a second check for collinearity using the Variance Inflation Factor (VIF) in multiple linear regression analysis. If the VIF values were below 10, we could consider that there was no collinearity between independent variables. All results were considered statistically significant at p<0.05. Two-sided 95% confidence intervals (CI) were calculated when appropriate. All calculations were performed with SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
Among the 390 patients recruited, patients who were studied for at least 3 days were considered to have completed this study whether they continued treatment with IV itraconazole or not. The final number of patients evaluated was 376. The clinical characteristics of the patients treated with empirical antifungal therapy with IV itraconazole are provided in Table 1. The median age was 48 years (range, 18-80 years). One hundred sixty-five patients were older than 50 and the male to female ratio was 1.1 : 1. Their underlying diseases are reported in Table 1: AML (n=229), ALL (n=55), MDS (n=23), NHL (n=52), HL (n=3), and MM (n=14). A total of 257 (68.4%) high-risk group of patients were included in this study. One hundred-three of 376 patients (27.4%) were in relapse or refractory status and 98 patients (26.1%) were enrolled during consolidation chemotherapy. Forty-six patients (12.2%) receiving HSCT were enrolled and 18 patients (39.1%) were included in the high-risk group. Twenty-one patients (5.6%) received an allogeneic HSCT. Among 284 patients with acute leukemia, 208 (73.2%) patients were considered high risk. There were 10 patients (43.5%) with MDS included in the high-risk group. Thirty-two patients (58.2%) with malignant lymphoma and 7 patients (50.0%) with MM were also considered as high risk (Table 1). There were 90 patients (23.9%) with co-morbidities. Of those, cardiovascular co-morbidity was reported in 42 patients (11.2%), diabetes mellitus in 20 (5.3%), liver disease in 19, lung disease in 14, renal disease in 4, and other medical conditions in 7 patients. At the initiation of empirical antifungal therapy, 123 patients (32.7%) had poor Eastern Co-operative Oncology Group (ECOG) performance status (2 or more).
Table 1.
Patient Characteristics
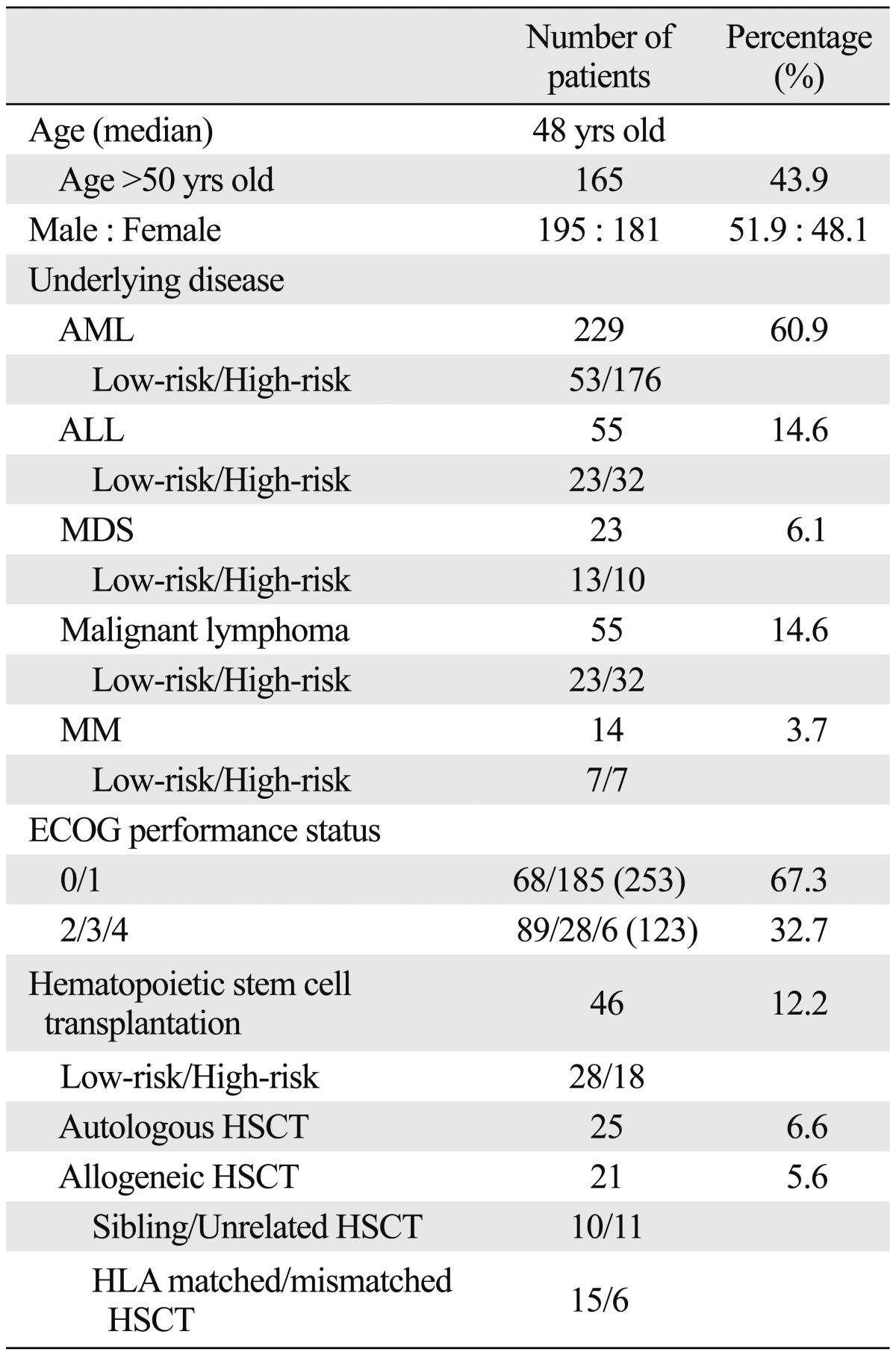
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; ECOG, Eastern Cooperative Oncology Group; HSCT, hematopoietic stem cell transplantation; HLA, human leukocyte antigen.
The results of baseline chest X-rays were available in 348 patients (92.6%). Among them, 70 patients (20.1%) showed abnormal findings on chest X-rays. Baseline high-resolution computerized tomography (CT) scan of the chest was performed in 59 patients (15.7%) and 51 patients (86.4%) had abnormal findings on CT. Sixteen patients (4.3%) had a compatible result for the diagnosis of IFD on the X-rays or CT. In 83 patients (22.1%) an enzyme immunoassay for serum galactomannan antigen was performed before initiation of empirical antifungal therapy. Among them, 9 patients (10.8%) had a positive result on the serum galactomannan test, a cut-off index of 0.5.26,27 The median duration of neutropenia prior to start of empirical antifungal therapy (baseline neutropenia) was 7 days (range, 1-173). Prolonged baseline neutropenia (more than 10 days) was observed in 32.4% of the patients (n=122). The median duration of neutropenic fever prior to initiation of empirical antifungal therapy (baseline neutropenic fever) was 3 days (range, 1-34).
About half of the patients had received an antifungal prophylaxis (n=183, 48.7%). The antifungal agents used for prophylaxis were itraconazole (n=69), fluconazole (n=86), nystatin (n=21), or D-AMB (n=7). The median duration of IV itraconazole for empirical antifungal therapy was 9 days (range, 3-28). Seventy-six patients (20%) switched from IV to oral itraconazole. Second-line antifungal therapy was conducted in 94 patients (25.0%) after discontinuation of empirical antifungal therapy with IV itraconazole. Antifungal agents for second-line therapy included D-AMB (n=84), L-AMB (n=1), caspofungin (n=7), fluconazole (n=1), or voriconazole (n=1). Seventy patients (18.6%) had documented systemic bacterial infections at baseline, and 13 patients (3.5%) had baseline fungal infections, including 11 Candida infections and two probable invasive aspergillosis infections, that were diagnosed within the first 48 hours after entry into this study.
Efficacy analysis
The overall success rate of empirical antifungal therapy with IV itraconazole was 51.3% in this study (Table 2). Among the 13 patients with baseline fungal infections, 7 patients (53.8%) had a successful outcome. Of the 11 baseline Candida infections, 7 patients achieved a successful outcome. The rate of occurrence of breakthrough fungal infections was 4.3%. Among 16 patients who developed breakthrough fungal infections, 8 patients (50.0%) had probable or proven invasive aspergillosis and 5 patients had infections with Candida species. The proportion of patients who survived for at least 7 days after completion of IV itraconazole therapy was 89.9% and the rate of resolution of fever during the neutropenic period was 70.2% (Table 2). The median time to defervescence after empirical antifungal therapy with IV itraconazole was 3 days (range, 1-22). Premature discontinuation of itraconazole therapy because of toxicity or lack of efficacy occurred in 34.8% (131/376).
Table 2.
Outcomes of Empirical Antifungal Therapy with Itraconazole
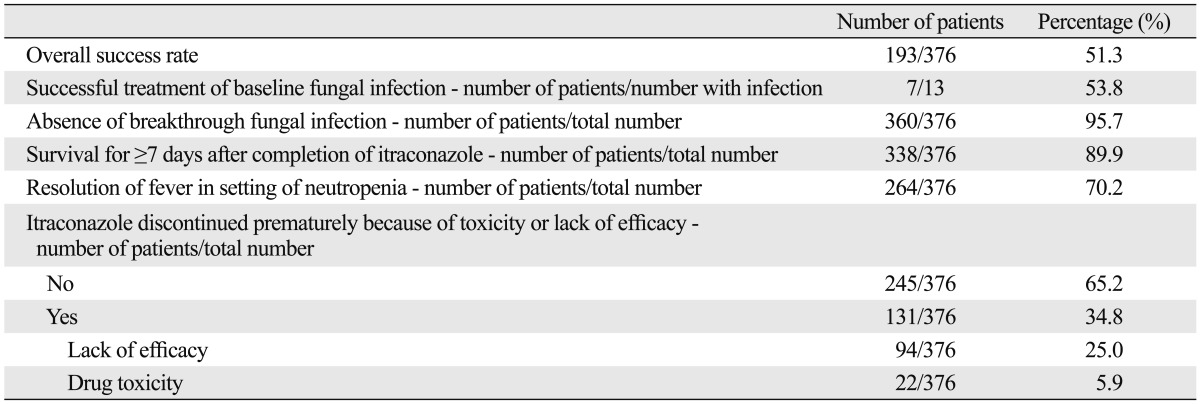
Baseline clinical characteristics and success rate of empirical antifungal therapy
We analyzed the effects of baseline clinical characteristics on the outcomes of empirical antifungal therapy with IV itraconazole. The patients over 50 years of age showed a lower success rate than the patients aged younger than 50 years (41.2% vs. 59.2%, p=0.001). The patients with acute leukemia had a success rate of 53.2% (151/284) and there were no significant differences in success rates according to the underlying hematological malignancies. Previous allogeneic HSCT was not significantly associated with a decreased success rate (57.1% vs. 51.0%, p=0.657) (Table 3). The patients classified into the high-risk group according to underlying tumor burden had a significantly lower success rate of empirical antifungal therapy than low-risk patients (47.5% vs. 59.7%, p=0.035). The patients with relapse or refractory acute leukemia had a lower success rate (44.6%, 33/74) than the patients with acute leukemia in CR (62.0%, p=0.036). The patients with co-morbidities were less likely to achieve a successful outcome in this study (41.2% vs. 54.5%, p=0.030) (Table 4). Among them, the patients with underlying lung disease showed a significantly lower success rate for empirical antifungal therapy (21.4% vs. 52.5%, p=0.028). Additionally, the overall success rate of empirical antifungal therapy with IV itraconazole was significantly decreased in the patients with a poor ECOG performance status (≥2) or evidence of IFD on X-rays or CT (p<0.001, p=0.009, respectively) (Table 4). There were no significant differences in the success rates according to the results of a baseline serum galactomannan test. Although the duration of baseline neutropenia was not correlated with the success rate, the patients with relatively early administration of empirical antifungal therapy (duration of neutropenic fever prior to start of empirical antifungal therapy ≤3 days) were more likely to achieve a successful outcome in this study (55.9% vs. 44.8%, p=0.037). Antifungal prophylaxis prior to starting empirical antifungal therapy did not significantly affect the success rate of empirical antifungal therapy (Table 4). However, the patients who had received an antifungal prophylaxis with itraconazole showed a significantly higher success rate than other patients (63.8% vs. 48.5%, p=0.024).
Table 3.
Outcomes of Empirical Antifungal Therapy According to Underlying Disease
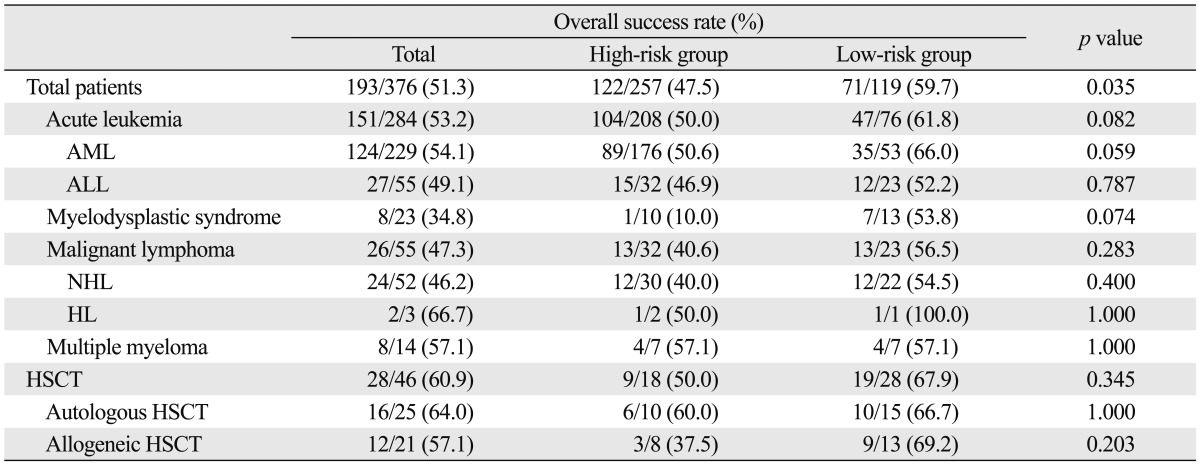
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin's lymphoma; HL, Hodgkin lymphoma; HSCT, hematopoietic stem cell transplantation.
Table 4.
Outcomes (Overall Success Rate) of Empirical Antifungal Therapy According to the Poor Prognostic Factors
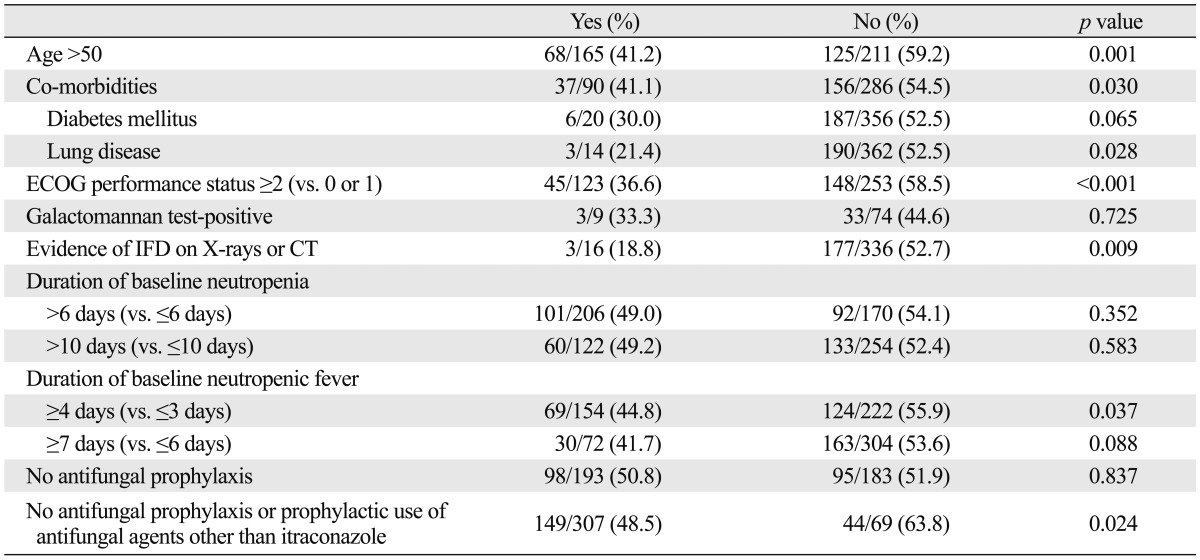
IFD, invasive fungal disease; ECOG, Eastern Cooperative Oncology Group; CT, computerized tomography.
In the present study, age over 50 years, underlying lung disease (co-morbidity), poor ECOG performance status (≥2), evidence of IFD on X-rays or CT, longer duration of baseline neutropenic fever (≥4 days), no antifungal prophylaxis or prophylactic use of antifungal agents other than itraconazole, and high tumor burden (high-risk group) were associated with a decreased success rate of empirical antifungal therapy with IV itraconazole in univariate analysis (Table 4). There was no collinearity between independent variables because all VIF values were below 10. Multivariate analysis using logistic regression revealed that age over 50 years [p=0.009, hazard ratio (HR)=1.81, 95% CI 1.16-2.82] and poor ECOG performance status (≥2) (p=0.005, HR=1.97, 95% CI 1.23-3.16) were significantly associated with a poor outcome of empirical antifungal therapy with IV itraconazole.
These two poor prognostic factors (age over 50 years and poor ECOG performance status) did not have a major impact on the rates of successful treatment of baseline fungal infection and development of breakthrough fungal infection. However, the patients older than 50 years of age or with a poor ECOG performance status (≥2) had a significantly lower survival rate for at least 7 days after discontinuation of empirical antifungal therapy (p=0.002, p=0.047, respectively). The patients older than 50 years of age or with a poor ECOG performance status (≥2) also had a significantly higher number of premature discontinuation of empirical antifungal therapy with IV itraconazole due to toxicity or lack of efficacy (p=0.017, p=0.001, respectively). Also, the patients over 50 years of age had a significantly lower rate of defervescence during the neutropenic period than the patients aged younger than 50 years (64.2% vs. 74.9%, p=0.031) (Table 5). The patients with a poor ECOG performance status (≥2) showed a lower trend of defervescence rate during neutropenic period (65.0% vs. 72.7%, p=0.149).
Table 5.
Comparison of the Success Rates of Empirical Antifungal Therapy in the Patients Who Presented with Poor Prognostic Factors
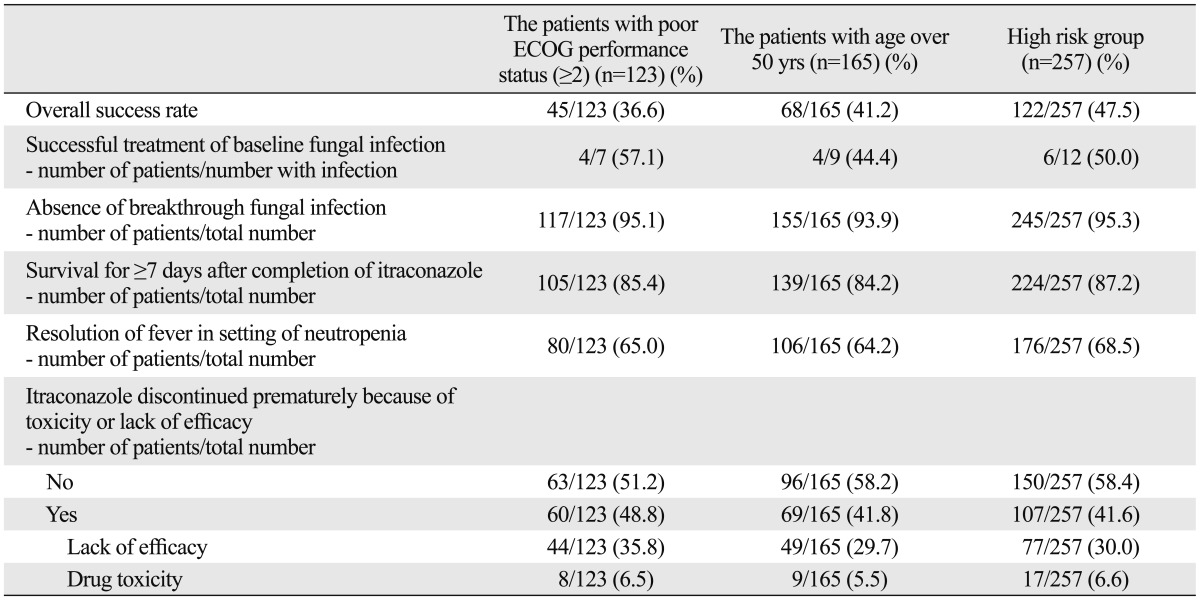
ECOG, Eastern Cooperative Oncology Group.
Safety and toxicities
Adverse events were reported in 114 patients (30.3%). Among them, hepatotoxicity (n=80, 21.3%), nephrotoxicity (n=27, 7.2%), and hypokalemia (n=22, 5.9%) were frequently observed. In 22 patients (5.9%), therapy with itraconazole had to be discontinued due to adverse events. The reported causes of withdrawal from itraconazole therapy due to toxicity were hepatotoxicity (n=17, 77.3%) and nephrotoxicity (n=4, 18.2%).
DISCUSSION
This prospective observational study, conducted in the patients with hematological malignancies at 26 medical centers, showed that empirical antifungal therapy with IV itraconazole was effective and safe compared with previous trials using IV itraconazole.6,7,12,13 We also identified prognostic factors for predicting the outcomes of empirical antifungal therapy in patients with recurrent or persistent neutropenic fever. Because numerous clinical trials for empirical antifungal therapy have demonstrated similar efficacies for several antifungal agents,4,6,11,22-24 it might not be necessary to further evaluate the differences in efficacy among antifungal agents. Because there are few reports on the prognostic factors for predicting the outcomes of empirical antifungal therapy with IV itraconazole,6,7,12,13 our analysis of the effect of potential prognostic factors on the outcomes of empirical antifungal therapy might be of value. In this study, age over 50 years and poor ECOG performance status (≥2) were deemed prognostic factors for predicting a poor outcome to empirical antifungal therapy with IV itraconazole.
Previously, patients with no antifungal prophylaxis or clinically documented infections showed a better outcome to empirical antifungal therapy in a trial with D-AMB.28 However, we were not able to use those results in clinical practice because this early trial did not use the current response criteria for empirical antifungal therapy, and physicians usually do not consider an empirical D-AMB therapy because of toxicity. Although prolonged neutropenia (>10 days) or allogeneic HSCT are commonly considered as important host factors for developing IFD,15,16 these two factors were not significant in our analysis. In our study, a poor outcome from empirical administration of IV itraconazole was observed in specific subgroups of patients: patients older than 50 years of age; patients with poor ECOG performance status (≥2), co-morbidities, especially lung disease, evidence of IFD on X-rays or CT, or a longer duration of baseline neutropenic fever (≥4 days); patients who did not receive antifungal prophylaxis with itraconazole (Table 4); and those who had a high tumor burden. Among them, age over 50 years and poor ECOG performance status (≥2) were shown to be poor prognostic factors in multivariate analysis. In the present study, patients over 50 years of age received empirical antifungal therapy with IV itraconazole at a median of 3 days after the onset of neutropenic fever (range, 1-21 days) and received a median of 8 days of IV itraconazole (range, 3-28 days). The patients with poor ECOG performance status (≥2) also received the empirical antifungal therapy for a sufficient duration (median 7 days). Therefore, we considered that these patients received an appropriate empirical antifungal therapy. However, they showed significantly lower success rates of empirical antifungal therapy because of low survival rates for at least 7 days after discontinuation of empirical antifungal therapy and high rates of premature discontinuation of empirical antifungal therapy. Therefore, we should consider a different strategy, such as a more potent or a combination antifungal therapy, in patients with these poor prognostic factors.
Although there were no significant results in multivariate analysis, several clinical characteristics were associated with a decreased overall success rate of empirical antifungal therapy with IV itraconazole in univariate analysis. Therefore, we suggest with caution the use of empirical antifungal therapy with IV itraconazole in patients with underlying lung disease (co-morbidity), evidence of IFD on X-rays or CT, longer duration of baseline neutropenic fever (≥4 days), no antifungal prophylaxis, prophylactic use of antifungal agents other than itraconazole, or high tumor burden. Although a positive galactomannan test is considered an important indicator of mycological infection,15,16,26 in our study, a positive baseline galactomannan test was not a significant poor prognostic factor. However, as radiological evidence of IFD was shown to be a poor prognostic factor in univariate analysis, we should re-evaluate the role of baseline galactomannan testing in the setting of empirical antifungal therapy, because the number of patients who had a result on baseline galactomannan test was very low (22.1%).
In this study, the patients with high tumor burden showed a decreased success rate of empirical antifungal therapy, despite a similar percentage of previous antifungal prophylaxis (51.4% in high-risk group, 51.3% in low-risk group). Because the patients receiving antifungal prophylaxis with itraconazole demonstrated better outcomes to empirical antifungal therapy with IV itraconazole, one might consider antifungal prophylaxis with itraconazole in high-risk patients to improve the results of empirical antifungal therapy with IV itraconazole. However, we, first, should confirm the role of IV itraconazole as an empirical antifungal therapy in future studies because there is little information available on the efficacy and safety of empirical antifungal therapy with IV itraconazole in patients who have received antifungal prophylaxis with itraconazole. If data on the blood concentration of itraconazole were available, we would have been able to explain the exact reason of the greater effectiveness of IV itraconazole in the patients with itraconazole prophylaxis, as we would have been able to observe low compliance with the itraconazole oral solution. A recent study comparing the effectiveness of the mold-active azole (voriconazole) versus fluconazole as an antifungal prophylaxis in patients undergoing HSCT suggested that intensive monitoring for IFD and early administration of appropriate empirical antifungal therapy is more important than the antifungal agent chosen for prophylaxis.29 Therefore, intensive work-up for identifying IFD and appropriate use of empirical antifungal therapy are more important in the management of patients with persistent neutropenic fever than appropriate use of antifungal prophylaxis with mold-active antifungal agents. In this study, we did not perform intensive serum galactomannan tests and radiographic monitoring in all patients for detecting IFD before initiation of empirical antifungal therapy (galactomannan test in 22.1% and high-resolution CT scan in 15.7%). We therefore assume that we would achieve better outcomes for empirical antifungal therapy if we perform a more aggressive work-up during neutropenic fever and determine an appropriate time for the initiation of empirical antifungal therapy according to the concept of preemptive therapy,5 especially in the patients with poor prognostic factors.
Our study has some limitations. Not all study sites selected the same initial antibiotics for neutropenic fever, as the trial was done in a more natural setting. We also performed this trial in a relatively small number of patients who had a prolonged neutropenia (32.4%) or received allogeneic HSCT (5.6%). However, the enrolled patients might be good candidates for analyzing prognostic factors because a sufficient number of patients (68.4%) belonged to the high-risk group and they all received the same antifungal agent (IV itraconazole) for empirical antifungal therapy. Because therapeutic drug monitoring (TDM) for evaluating sufficient blood concentrations of itraconazole is important,30 we aim to develop a protocol with regular evaluation of TDM for a future trial.
In conclusion, this prospective multicenter observational study demonstrated that empirical antifungal therapy with IV itraconazole is effective and safe in Korean patients with hematological malignancies. Since patients older than 50 years of age or with poor performance status at the time of initiation of empirical antifungal therapy showed poor outcomes from empirical antifungal therapy, we propose that a more aggressive work-up for identifying mycological evidence of IFD, including serum galactomannan test and high-resolution CT, should be required in these patients. In the future, it may be helpful to distinguish which patients would benefit most from empirical antifungal therapy and determine the optimum time of initiation for empirical antifungal therapy during neutropenic fever.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from Janssen Korea, Seoul, Korea.
We would like to thank all the nurses and doctors involved in caring for the patients. In addition to the authors, the following investigators participated in the study: Min Kyoung Kim (Yeungnam University College of Medicine, Daegu); Seok Jin Kim (Sungkyunkwan University School of Medicine, Seoul); Chul Won Jung (Sungkyunkwan University School of Medicine, Seoul); Hun-Mo Ryoo (Daegu Catholic University School of Medicine, Daegu); Yeung Chul Mun (Ewha Womans University, Seoul); Hwa Jung Sung (Korea University College of Medicine, Seoul); Hyeon Seok Eom (National Cancer Center, Goyang); Gyeong-Won Lee (Gyeongsang National University, Jinju); Moon Hee Lee (Inha University Hospital, Incheon); Jeong Lim Lee (Daegu Fatima Hospital, Daegu); and Deog-Yeon Jo (Chungnam National University Hospital, Daejeon).
Footnotes
This study was presented at the 4th Advances Against Aspergillosis, Rome, Italy, February 4-6, 2010.
The authors have no financial conflicts of interest.
References
- 1.Upton A, Marr KA. Emergence of opportunistic mould infections in the hematopoietic stem cell transplant patient. Curr Infect Dis Rep. 2006;8:434–441. doi: 10.1007/s11908-006-0017-5. [DOI] [PubMed] [Google Scholar]
- 2.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–1075. [PubMed] [Google Scholar]
- 3.Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95:644–650. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis. 2004;39(Suppl 1):S38–S43. doi: 10.1086/383052. [DOI] [PubMed] [Google Scholar]
- 5.Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, Suarez F, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48:1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 6.Boogaerts M, Winston DJ, Bow EJ, Garber G, Reboli AC, Schwarer AP, et al. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy. A randomized, controlled trial. Ann Intern Med. 2001;135:412–422. doi: 10.7326/0003-4819-135-6-200109180-00010. [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Min WS, et al. Intravenous itraconazole vs. amphotericin B deoxycholate for empirical antifungal therapy in patients with persistent neutropenic fever. Korean J Intern Med. 2006;21:165–172. doi: 10.3904/kjim.2006.21.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 9.Maertens J, Marchetti O, Herbrecht R, Cornely OA, Flückiger U, Frêre P, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 update. Bone Marrow Transplant. 2011;46:709–718. doi: 10.1038/bmt.2010.175. [DOI] [PubMed] [Google Scholar]
- 10.Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011;26:220–252. doi: 10.3904/kjim.2011.26.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klastersky J. Antifungal therapy in patients with fever and neutropenia--more rational and less empirical? N Engl J Med. 2004;351:1445–1447. doi: 10.1056/NEJMe048203. [DOI] [PubMed] [Google Scholar]
- 12.Schuler U, Bammer S, Aulitzky WE, Binder C, Böhme A, Egerer G, et al. Safety and efficacy of itraconazole compared to amphotericin B as empirical antifungal therapy for neutropenic fever in patients with haematological malignancy. Onkologie. 2007;30:185–191. doi: 10.1159/000100055. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, Nishiki Kosaka S, Nakao Y, Kumura T, Hagihara K, Sakamoto E, et al. Efficacy and safety of intravenous itraconazole as empirical antifungal therapy for persistent fever in neutropenic patients with hematological malignancies in Japan. Int J Hematol. 2009;89:649–655. doi: 10.1007/s12185-009-0316-3. [DOI] [PubMed] [Google Scholar]
- 14.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 16.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 18.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 19.Borchmann P, Eichenauer DA, Engert A. State of the art in the treatment of Hodgkin lymphoma. Nat Rev Clin Oncol. 2012;9:450–459. doi: 10.1038/nrclinonc.2012.91. [DOI] [PubMed] [Google Scholar]
- 20.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 21.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 23.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 24.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 25.Common terminology criteria for adverse events (CTCAE) v3.0. National Cancer Institute; 2006. [Google Scholar]
- 26.Maertens J, Verhaegen J, Lagrou K, Van Eldere J, Boogaerts M. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood. 2001;97:1604–1610. doi: 10.1182/blood.v97.6.1604. [DOI] [PubMed] [Google Scholar]
- 27.Maertens JA, Klont R, Masson C, Theunissen K, Meersseman W, Lagrou K, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis. 2007;44:1329–1336. doi: 10.1086/514349. [DOI] [PubMed] [Google Scholar]
- 28.EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989;86(6 Pt 1):668–672. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 29.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasmacher A, Hahn C, Leutner C, Molitor E, Wardelmann E, Losem C, et al. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses. 1999;42:443–451. doi: 10.1046/j.1439-0507.1999.00505.x. [DOI] [PubMed] [Google Scholar]


