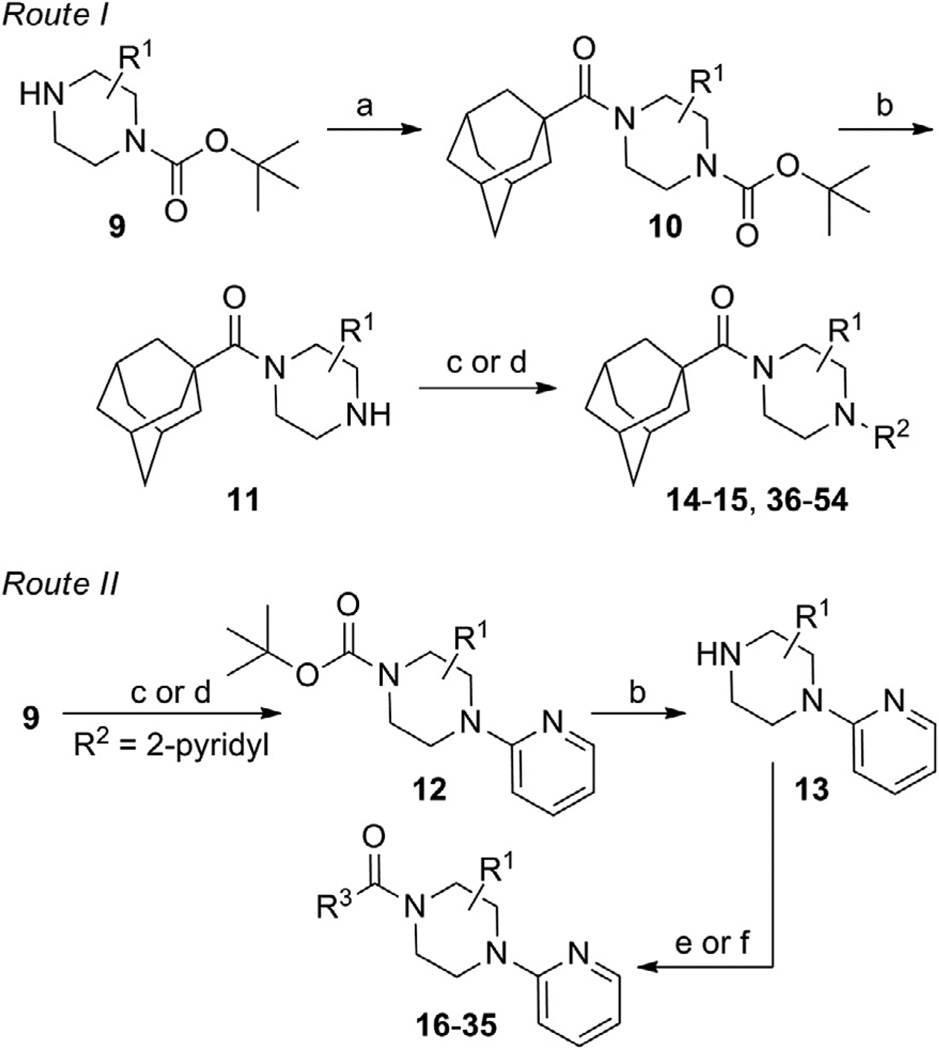
Scheme 1.
Reagents and conditions: (a) 1-adamantoyl chloride, DIEA, CH2Cl2; (b) HCl, MeOH, dioxanes; (c) R2F, NMP, μ wave, 250 °C, 10 min; (d) R2X (X = Cl, Br, or I), Pd2(dba)3 or Pd(OAc)2, Xantphos, NaOtBu or Cs2CO3, dioxanes, μ wave, 120 °C, 10 min or 100 °C, 18 h; (e) R3COCl, DIEA, CH2Cl2; (f) R3CO2H, HATU, DIEA, CH2Cl2.