Figure 1. Fe limitation and Fe acquisition during bacterial infections.
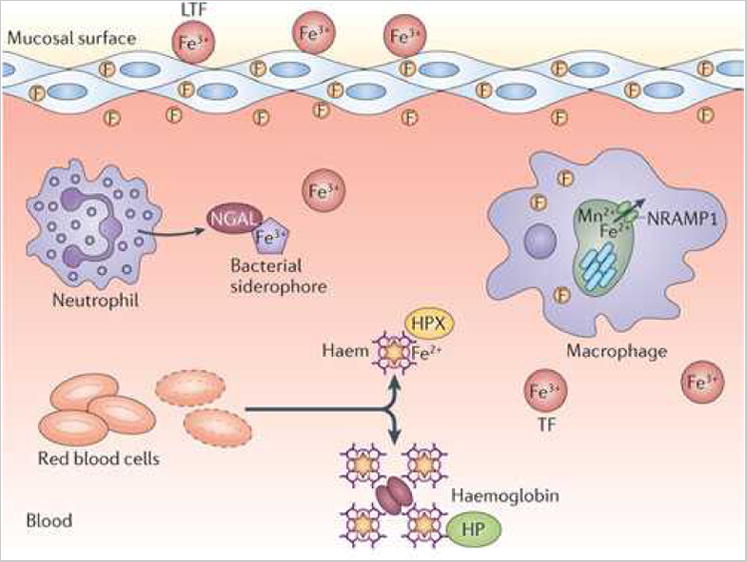
a. Overview of Fe limitation strategies in the vertebrate host. Fe3+ is stored intracellularly in complex with ferritin (F), bound by serum transferrin (TF) or bound by lactoferrin (LTF) at mucosal surfaces. In the blood, Fe2+ is complexed with haem, which is bound by haemoglobin within red blood cells (RBCs). Upon red cell lysis, haemoglobin is bound by haptoglobin (Hpt) and free haem is scavenged by haemopexin (Hpx). In addition to these haem-binding proteins, neutrophil gelatinase-associated lipocalin (NGAL) binds and sequesters bacterial siderophores. b. Representative Fe acquisition systems expressed by Gram-negative and Gram-positive pathogens. Both Gram-negative and Gram-positive pathogens possess systems to acquire Fe3+-siderophores, Fe2+-haem, Fe3+ from transferrin and/or free Fe2+. Not all systems are expressed by the same organism. TF, transferrin; SIP, siderophore interacting protein; HO, haem oxygenase; Hpt, haptoglobin; OM, outer membrane; P, periplasm; IM, inner membrane; CW, cell wall; CM, cytoplasmic membrane.
