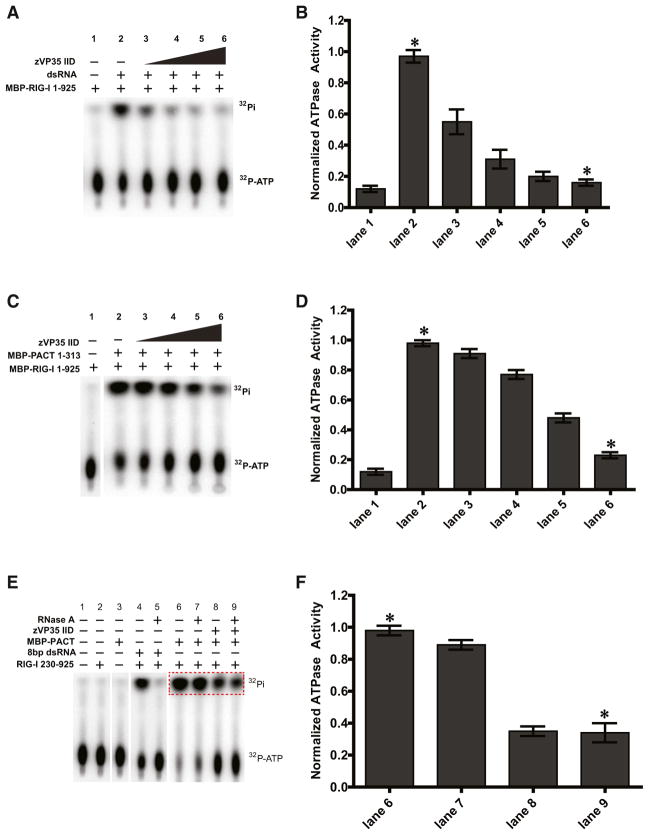
Figure 4. VP35 Inhibition of RIG-I ATPase Activation Is dsRNA Independent.
(A and C) VP35 inhibits activation of RIG-I ATPase activity by dsRNA (A) and PACT (C) in a concentration-dependent manner.
(B and D) Quantitation of ATPase activity, represented by the amount of inorganic phosphate in each lane, was normalized to lane 2. The data represent the mean, and error bars indicate the standard deviation. The difference between the indicated groups was found to significant as indicated by *p = 0.0015 (B) and p = 0.0007 (D).
(E) Inhibition of PACT-mediated RIG-I activation by VP35 is RNA independent. Addition of RNase A abolishes RIG-I activation by dsRNA (lane 4 versus lane 5), but RNase A has no impact on the VP35 inhibition of PACT (compare lane 8 versus lane 9). Free inorganic phosphate levels were quantified for the region highlighted by the red dotted box.
(F) Quantitation of relative ATPase activity for lanes 6–9 normalized to ATPase activity of lane 6. The difference between the indicated groups was found to be significant, *p = 0.0054. The data represent the mean of three independent experiments with the error bars indicating the standard deviation. See also Figure S2.