Table 1.
Cascade amination/amidation: Catalyst identification.[a]
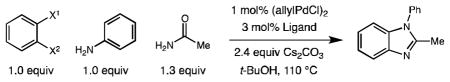
| ||||
|---|---|---|---|---|
| Entry | X1 | X2 | Ligand | Yield[b] |
| 1 | OTf | Cl | PPh3 | 0% |
| 2 | OTf | Cl | PCy3 | 0% |
| 3 | OTf | Cl | PtBu3 | 0% |
| 4 | OTf | Cl | L1 | trace |
| 5 | OTf | Cl | L2 | 47% |
| 6 | OTf | Cl | L3 | 63% |
| 7 | OTf | Cl | L4 | 77% |
| 8 | OTf | Cl | 2 mol% P1[c] | 88% (86%) |
| 9 | Br | Cl | 2 mol% P1[c] | 87% |
| 10 | Cl | Cl | 2 mol% P1[c] | 70% |
Reaction conditions: aryl halide (0.5 mmol), aniline (0.5 mmol), acetamide (0.65 mmol), (allylPdCl)2 (3 mol%), ligand (3 mol%), Cs2CO3 (1.2 mmol), tBuOH (1.0 mL), 110 °C, 12 h.
Yield determined by GC using tetradecane as internal standard, isolated yield in parenthesis.
Precatalyst P1 was used instead of (allylPdCl)2 and ligand.
![[c]](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c7b5/3875363/264190208e99/nihms534443u4.jpg)
