Abstract
OBJECTIVE
Cancer patients and partners often report inadequate communication about illness-related issues, although it is essential for mutual support and informal caregiving. This study examined the patterns of change in dyadic communication between patients with prostate cancer and their partners, and also determined if certain factors affected their communication over time.
METHOD
Using multilevel modeling, this study analyzed longitudinal data obtained from a randomized clinical trial with prostate cancer patients and their partners, to examine their communication over time. Patients and partners (N=134 pairs) from the usual-care control group independently completed baseline demographic assessment and measures of social support, uncertainty, symptom distress, and dyadic communication at baseline, and 4-, 8-, and 12-month follow-ups.
RESULTS
The results indicated that (1) patients and partners reported similar levels of open communication at the time of diagnosis. Communication reported by patients and partners decreased over time in a similar trend, regardless of phase of illness; (2) phase of illness affected couples’ open communication at diagnosis but not patterns of change over time; and (3) couples’ perceived communication increased as they reported more social support, less uncertainty, and fewer hormonal symptoms in patients. Couples’ demographic factors and general symptoms, and patients’ prostate cancer-specific symptoms did not affect their levels of open communication.
CONCLUSIONS
Perceived open communication between prostate cancer patients and partners over time is affected by certain baseline and time-varying psychosocial and cancer-related factors. The results provide empirical evidence that may guide the development of strategies to facilitate couples’ interaction and mutual support during survivorship.
INTRODUCTION
Open communication between patients and partners about cancer-related issues is an important resource when they cope with the demands of cancer and the side effects of treatment [1-2]. More cancer-related open communication has been associated with greater mutual support [3-4], higher quality of life (QOL), better psychosocial adjustment, and higher relationship functioning in both partners [5-9]. Communication of cancer-related feelings, views, and problems that is mutually open between partners has stronger protective effects on psychological well-being when cancer patients experience more physical impairment [6].
On the contrary, lack of exchanging cancer-related concerns compromises patient-partner relationships and their psychological adjustments [7, 10]. These negative effects can be exacerbated when couples have discordant communication patterns (i.e., limited disclosure by one spouse and more open for the other spouse) [6, 11]. Lack of open communication about cancer-related issues harms the intimate relationship and psychosocial well-being even when one or both partners hide concerns and feelings in an attempt to protect themselves and/or their loved one [8-9]. Problems communicating are more detrimental when couples have satisfied relationships prior to a cancer diagnosis [8-9].
Communication between partners managing prostate cancer is especially important. Known as a “couple’s illness,” prostate cancer and its treatment side-effects negatively affect couples’ intimate relationships because of the deteriorating physical symptoms (e.g., incontinence and impotence) [12-14], psychological distress (e.g., uncertainty, anxiety, and fear) [15-18], and financial difficulties [18-22]. The relationship context has been recognized as important during couples’ adjustment to prostate cancer [21, 23]. Yet, research indicates that maintaining effective relationships is one of the major challenges couples face [24-25]. In a recent study, about 40% of the patients reported a significant decline in the quality of their marital relationship as a consequence of prostate cancer [22]. The lack of open spousal communication can further strain couple’s relationship function [26]. Yet, studies on open communication in the context of cancer have focused primarily on couples managing non-gender-related [7, 11] or female-specific cancers [6, 9, 27-28]; communication issues that are related to masculinity and intimate relationships in the context of cancer survivorship are rarely addressed. Given the fact that prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer deaths in men in the U.S. [29], it is imperative to understand how patients interact within their social context, especially how they exchange cancer-related information with the partner during survivorship.
Open communication about cancer-related issues is also important because of the major role that spouses play in patients’ lives. Prostate cancer is most prevalent among men 65 years of age and older [30-31]. As people age, their social networks shrink due to retirement, illness and death of family members and acquaintances, or their declining personal health status [32]. The diagnosis of prostate cancer, especially some of the symptoms (e.g., incontinence) further decreases their socialization [33]. While they primarily interact with their own families, men with prostate cancer identify spouses as their health monitors, caregivers, and the major (sometimes the only) source of support, especially emotional support [34-36].
Although research has found that open communication helps partners reconnect with each other in the face of physical and emotional adversity [37], couples often experience difficulties in communication while managing prostate cancer [33, 35-36, 38]. Wives identify patients’ lack of communication as one of the most common challenges [33, 39]: patients hide symptoms and treatment side effects, and their negative effects on emotional well-being [17, 33]. When patients have poor sexual function, partners are more likely to report avoidance of open spousal communication [26]. Partners avoid communication to protect each other from the negative feelings (e.g., fears and uncertainty) or to protect themselves from the reactions they might receive from the other partner [18, 35, 39]. Yet, wives find men’s protective efforts to be antagonistic, especially when men’s attempts to hide negative feelings are unsuccessful [37].
It is also important to understand how couples exchange cancer-related information over time. The diagnosis of cancer is not a single event; cancer survivorship is characterized by a series of multiple, interwoven, and layered psychosocial transitions that affects both the patient and his family members [1, 40]. During the continual psychosocial adaptation throughout cancer survivorship, partners constantly modify their coping efforts as they face different challenges [41]. A longitudinal study by Gray and colleagues, using qualitative approach, found that couples’ open communication decreased after prostate cancer treatment was completed [38]. Yet, most studies about communication issues in the context of prostate cancer have been retrospective and/or cross-sectional [10, 26]. Research from a longitudinal perspective, however, can help examine how partners join their coping resources through information exchange, and how social contextual factors contribute to the patterns of change in open communication during the survivorship trajectory. This information can provide guidance for developing strategies to promote effective interaction and mutual support between partners.
Factors related to communication
The Stress-Appraisal theory was used to examine certain factors that may be related to couples’ communication when coping with cancer [42-44]. According to this theory, there are personal, social/family, and cancer-related factors that influence how people manage the demands of illness and maintain their well-being. For this study, personal factors were operationalized as demographic factors, social/family factors as social support and the length of relationship, and cancer-related factors as time since diagnosis, phase of illness, symptom distress, and uncertainty about the illness. Open dyadic communication, the dependent variables, was defined as the levels of exchange of cancer-related information, feelings, and concerns between patients with prostate cancer and their partners. Due to the lack of literature about communication issues in the area of prostate cancer, we have supplemented the following section with research results with other cancer populations.
Among personal factors, older age has been negatively associated with less open communication in patients with early stage breast cancer [27-28] or in cancer patients receiving chemotherapy [45]. Research about the relationships between other personal factors (e.g., gender, education level) and couples’ cancer-related communication is limited and inconclusive [7, 33, 46]. Regarding social/familial factors, research in couples facing breast cancer has shown that more social support from friends was related to less open communication between partners [28].
Among cancer-related factors, time since diagnosis has been shown to negatively affect couples’ communication in cross-sectional and retrospective studies. Couples’ cancer-related communication decreased over time [7], especially upon the completion of treatments [7, 38]. Relationships between phases of illness and couples’ communication have been inconsistent [7, 27]. Nonetheless, couples facing biochemical recurrent prostate cancer are found to have less open communication about the illness than couples with newly diagnosed localized or advanced cancer [42]. Uncertainty about the illness, common among cancer patients and partners [42], has been linked to more communication between cancer patients and health professionals [47-49]. Yet, little research has explored how uncertainty affects communication about cancer-related issues between partners. Although qualitative studies have found that cancer patients and spouses had trouble discussing cancer-related symptoms (especially sexual functions), prognosis, and the emotional effects of cancer [7, 26, 33, 50], there is limited research on how cancer symptom distress affects couples’ cancer-related communication.
The purpose of this longitudinal study was to examine the patterns of change in perceived dyadic communication over time and the longitudinal relationship between selected factors and couples’ communication during prostate cancer. The specific aims were two-fold: (1) to compare patterns of change in levels of dyadic communication over time by role (patient versus partner) and by phase of illness (i.e., localized, recurrent, and advanced); and (2) to examine whether personal, social/familial, and cancer-related factors affected dyadic communication between patients and partners over time.
METHODS
Study Design and Sample
This was a secondary analysis of the longitudinal data obtained from a randomized controlled trial (RCT) that examined the effects of a family-based intervention on the QOL of prostate cancer patients and their partners [2]. The sample for the RCT consisted of 236 couples. Prostate cancer patients were eligible if in one of three phases of illness: localized, biochemical recurrence, or advanced. Patients were treated with a prostatectomy or external beam radiation with or without hormonal treatments and/or chemotherapy. More details about the original sample and procedures have been reported previously [2, 42, 51]. This secondary analysis included 134 pairs of patients with prostate cancer and partners from the usual care control group to eliminate any effects of the experimental intervention on the study variables. Patients and partners independently completed assessment at 4 data points: baseline, and 4-, 8-, and 12-month follow-ups.
Measurement
Communication
The outcome variable, level of perceived open dyadic communication about cancer-related issues, was measured with the 23-item Lewis Mutuality and Interpersonal Sensitivity Scale (MIS) [52]. The MIS uses a 5-point Likert scale ranging from 1 (low) to 5 (high), with higher scores indicating that patients and partners perceive more open communication about cancer-related issues. Examples of items include “We keep the communication open between us about the cancer” and “We spend a lot of time talking about how things are going with the cancer.” Construct and criterion validity was established among breast cancer patients and partners [52]. Communication was assessed at baseline, 4, 8, and 12 months later. The internal consistency reliability ranged from .90 to .94 across the four data points for patients and spouses.
Demographic factors (age and education) and family factors (income and length of relationship) were obtained at baseline using the demographic section of the Risk for Distress (RFD), previously referred to as the Omega Screening Questionnaire [53].
Social support was assessed at baseline and follow-ups with the Personal Resource Questionnaire (PRQ) developed by Brandt and Weinert [54]. This 25-item Likert scale measures the amount of perceived support from others (e.g., friends and relatives). Higher scores indicate more support. In this study, the internal reliability coefficients for patients and spouses ranged from .88 to .93 across 4 follow-up time points.
Among cancer-related factors, months since diagnosis and phase of illness (localized, recurrent, and advanced) were obtained at baseline from patients’ medical history questionnaire. Uncertainty about the illness was assessed at all data points using the 28-item Mishel Uncertainty in Illness Scale [55]. Higher scores indicated more uncertainty. Validity and reliability of this scale have been well established in cancer patients [55-56]. The internal reliability coefficients for patients and spouses ranged from .91 to .94 across 4 assessment time points in this study.
Symptom Distress included prostate cancer-specific symptoms and general symptoms, and were assessed at baseline and follow-ups. Prostate cancer-specific symptoms in patients (i.e., bowel, hormonal, sexual, or urinary symptoms) were measured using the 50-item Expanded Prostate Cancer Index Composite (EPIC) [57]. Higher scores indicate fewer symptoms. Reliability and validity of the EPIC are well established [42, 57]. Cronbach alphas for EPIC subscales ranged from .74 to .90 across 4 time points in this study. The partners completed a four-item EPIC (spousal version) which assessed how much of a problem their husbands’ bowel, hormonal, sexual, or urinary symptoms was for the spouses. General symptoms (e.g., fatigue, pain, and insomnia) were measured with the 16-item Symptom Subscale of the Risk for Distress Scale [53]. Patients and partners independently rated their own symptoms on a 3-point scale. The psychometric properties of this scale has been tested in patients with various types of cancer and family caregivers [42, 53, 58]. Higher scores indicate more general symptoms. In this study, the internal consistency coefficients ranged from .76 to .84 for patients and partners across 4 time points of assessment.
Data Analysis
Preliminary descriptive analyses were conducted for couples’ demographic and medical characteristics. To achieve research aims, multilevel modeling (MLM) for longitudinal measures was employed using maximum likelihood estimation [59] in SAS 9.2 PROC MIXED [60]. The MLM specification conceptualizes that the time-varying measures were nested within individuals and individuals nested within couples. Thus, the variability in couples’ communication is partitioned into 3 levels (Figure 1): intra-personal, intra-couple, and inter-couple.
Figure 1.
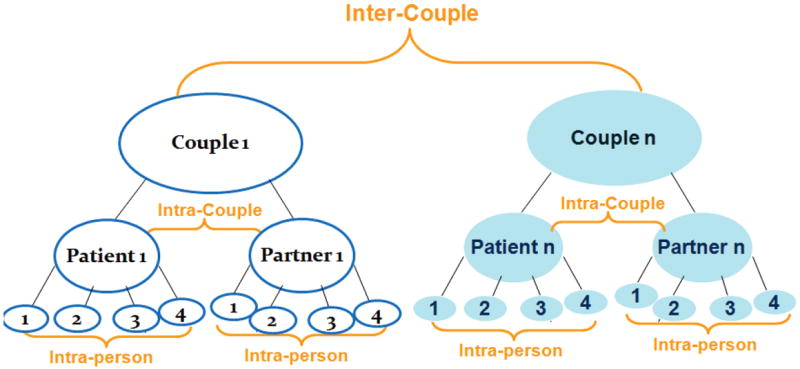
Data Levels for the Multilevel Models for Couples
A series of multilevel models were fitted. The Initial Model included three variables: time (intra-personal), role (patient vs partner, intra-couple), and phase (localized, recurrent, and advanced, inter-couple), and their interactions. The Full Model, which included baseline and time-varying personal, psychosocial, and cancer-related factors, was then fitted to examine whether the relationships between the predictors and outcomes in the Initial Model changed when controlling for selected covariates. Finally, the parsimonious Final Model was specified by eliminating the insignificant interactions and covariates from the full model so that predictors that significantly affected the changes in couples’ communication across time could be analyzed.
The “time” predictor, treated as a continuous variable, was calculated as the months since diagnosis at baseline (which varied for different patients) plus the months since baseline of the follow-up assessments (i.e., 0, 4, 8, and 12, respectively). The linear and quadratic effects of time were included in model specification to capture the potential curvilinear pattern of communication over time.
Competing models were compared via (1) the Akaike’s information criterion (AIC) and Bayesian information criterion (BIC), (2) the likelihood ratio test (LRT)—a statistical test comparing the fit of a larger model to that of a nested model with fewer parameters [59], and (3) the effect size—the percentage of change in the variance components.
RESULTS
Descriptive Findings
Of the 134 patient-spouse pairs in the control group who completed the baseline assessment, 124, 123, and 114 pairs completed the follow-up assessments at 4, 8, and 12 months, respectively. About 84% of patients and partners were white and 13% were African Americans. Patients were diagnosed with localized (65%), biochemical recurrent (12%), or advanced cancer (23%). Couples in the localized group were younger and had more recent diagnosis than those with biochemical recurrent and advanced cancers (Table 1). Compared to their partners, patients were older and had higher education.
Table 1.
Demographic and Medical Characteristics among Patients and Spouses at Baseline
| Variable | Phase of Illness
|
Overall (N=134 pairs) | F Ratio (Phase Effect) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Localized (N=87 pairs) | Recurrent (N=16 pairs) | Advanced (N=31 pairs) | ||||||||
|
| ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (Years) | Patient | 60.4 | 8.3 | 67.8 | 10.1 | 65.9 | 9.6 | 62.6 | 9.2 | 7.66*** |
| Spouse | 57.2 | 8.5 | 64.6 | 11.8 | 60.8 | 10.3 | 58.9 | 9.7 | 4.99*** | |
|
| ||||||||||
| Education (Years) | Patient | 16.5 | 3.8 | 15.4 | 3.4 | 15.4 | 3.2 | 16.1 | 3.6 | 1.45 |
| Spouse | 15.3 | 2.5 | 13.8 | 2.1 | 13.5 | 3.1 | 14.7 | 2.7 | 6.28** | |
|
| ||||||||||
| Length of Marriage (Years) | 30.2 | 13.1 | 33.3 | 17.2 | 35.4 | 15.6 | 31.7 | 14.3 | 1.59 | |
|
| ||||||||||
| Time since Diagnosis (Months) | 7.9 | 4.2 | 85.3 | 42.2 | 58.9 | 46.0 | 29.0 | 39.7 | 83.91*** | |
|
| ||||||||||
| Family Income | % | % | % | % | ||||||
| ≤ $15,00 | 2.5 | 21.4 | 10.0 | 6.5 | ||||||
| $15,001-$30,000 | 11.4 | 42.9 | 40.0 | 22.0 | 30.65*** | |||||
| $50,001 - $75,000 | 17.7 | 28.6 | 16.7 | 18.7 | ||||||
| >$75,000 | 68.4 | 7.1 | 33.3 | 52.8 | ||||||
p<.05;
p<.01;
p<.001.
Model Fitting Results (Table 2)
Table 2.
Parameter Estimates for Multilevel Models of Couples’ Communication
| Effect | Initial Model | Full Model | Final Model | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| β | SE | β | SE | β | SE | ||
|
| |||||||
| Intercept | 3.75*** | 0.14 | 3.74*** | 0.35 | 3.83*** | 0.11 | |
|
| |||||||
| ROLE [Reference: Spouse (SP)] | Patient (PT) | 0.10 | 0.13 | 0.10 | 0.12 | 0.12 | 0.06 |
|
| |||||||
| Phase of Illness (Reference: Advanced) | Localized | -0.52** | 0.17 | -0.51** | 0.17 | -0.50*** | 0.13 |
|
| |||||||
| Recurrent | 0.23 | 0.30 | 0.17 | 0.29 | -0.09 | 0.17 | |
|
| |||||||
| Age_PT | -0.004 | 0.01 | |||||
|
| |||||||
| Age_SP | 0.01 | 0.01 | |||||
|
| |||||||
| Education_PT | -0.003 | 0.02 | |||||
|
| |||||||
| Education_SP | -0.01 | 0.02 | |||||
|
| |||||||
| Income | . | -0.004 | 0.06 | ||||
|
| |||||||
| Length of Relationship | . | 0.003 | 0.01 | ||||
|
| |||||||
| Time | -0.01** | 0.004 | -0.01* | 0.004 | -0.01*** | 0.002 | |
|
| |||||||
| Time_Squared (Time_sq) | 0.0001ˆ | 0.00004 | 0.00005 | 0.00004 | 0.0001*** | 0.00003 | |
|
| |||||||
| Social Support[a] | 0.01*** | 0.001 | 0.01*** | 0.001 | |||
|
| |||||||
| Uncertainty[b] | -0.008*** | 0.001 | -0.01*** | 0.001 | |||
|
| |||||||
| Prostate Cancer Specific Symptoms[a] | Urine | -0.006 | 0.02 | ||||
|
| |||||||
| Bowel | -0.006 | 0.02 | |||||
|
| |||||||
| Sexual | 0.003 | 0.01 | |||||
|
| |||||||
| Hormonal | 0.06** | 0.02 | 0.07*** | 0.02 | |||
|
| |||||||
| General Symptoms[b] | -0.01 | 0.01 | |||||
|
| |||||||
| Role*Phase (Reference: Pt*Advanced and SP*all phases | PT*Localized | 0.17 | 0.17 | 0.08 | 0.16 | ||
|
| |||||||
| Pt*Recurrent | -0.25 | 0.21 | -0.31 | 0.19 | |||
|
| |||||||
| Time*Role (Reference: Time*SP) | Time*PT | 0.01** | 0.003 | 0.01* | 0.003 | 0.005ˆ | 0.003 |
|
| |||||||
| Time_sq*Role (Reference: Time_sq*SP) | Time_sq*PT | -0.0001* | 0.00003 | -0.0001 | 0.00003 | -0.00004 | 0.00003 |
|
| |||||||
| Time*Phase (Reference: Time*Advanced) | Time*Localized | -0.01 | 0.01 | -0.01 | 0.01 | ||
|
| |||||||
| Time*Recurrent | -0.01 | 0.01 | -0.01 | 0.01 | |||
|
| |||||||
| Time_sq*Phase (Reference: Time_sq*Advanced) | Time_sq*Localized | -0.0002 | 0.0003 | 0.00004 | 0.0003 | ||
|
| |||||||
| Time_sq*Recurrent | 0.00005 | 0.0001 | 0.0001 | 0.0001 | |||
|
| |||||||
| Fit Statistics: | -2 Log Likelihood | 1224.7 | 988.8 | 997.7 | |||
| AIC (smaller is better) | 1260.7 | 1050.8 | 1027.7 | ||||
| BIC (smaller is better) | 1312.9 | 1137.2 | 1069.5 | ||||
Denotes
p<.05;
p<.01;
p<.001;
p=.06
Higher scores indicate more positive results: more support from others and less Pca specific symptoms
Higher scores indicate more negative results: more uncertainty and more general symptoms
Results of the Initial Model showed that, compared to couples facing advanced cancer, those with recurrent cancer had similar levels of communication, and those with localized cancer perceived significantly less communication at diagnosis (p<.01). The results of time linear (p<.01) and squared (p=.06) effects indicated that couples’ perceived communication decreased over time in a somewhat curvilinear trend. The significant interactions between time (linear and squared) and role (p<.01 and p<.05, respectively) suggested that the observed patterns of change in communication varied by role (i.e., patient vs. spouse). Patients’ perceived level of open communication decreased at a slower speed than their partners. Role main effect was insignificant when no variables other than time and phases of illness were considered.
In the Full Model that controlled for all baseline and time-varying covariates, the relationships demonstrated in the Initial Model remained stable except that the time-squared terms became insignificant. Greater social support (p<.001), less uncertainty (p<.001), and fewer prostate cancer-specific hormonal symptoms (p<.01) were significantly related to more perceived communication. After excluding insignificant interactions and covariates, the Final Model was obtained. The main effects that were significant in the initial model and the full model remained significant; the trajectory of change in the levels of open dyadic communication about cancer was marginally different between patients and partners (p=.06).
Regarding model integrity evaluation, the comparison of AICs and BICs of full and final models supported the final model. The likelihood ratio test results (chi-sq=8.9, df=22, p=.99, Nobservation=872) also suggested that the final model, although having fewer predictors, has a goodness-of-fit that is similar to that of the full model. The significant random effects in the final model indicated that the variability in couples’ communication was partitioned into three parts: 26% at intra-personal, 32% at intra-couple, and 42% at inter-couple levels, after controlling for the fixed effects of psychosocial and symptom covariates.
Finally, compared to the initial model which only included time-invariant variables (i.e., time, phase, role, and their interactions), the final model which also included time-varying predictors (i.e., social support, uncertainty, and prostate cancer-specific hormonal symptoms) improved variance estimates. The final model reduced the variance in couples’ communication by 15.3% at the intra-personal level, 28.2% at the intra-couple level, and 11.4% at the inter-couple level.
Summary of Results Addressing Research Aims
Aim 1
Patients and partners reported similar levels of perceived communication at diagnosis when adjusting for selected covariates. Communication reported by patients and partners decreased over time in a similar trend, regardless of phase of illness.
Perceived dyadic communication at diagnosis varied by phase of illness. Patients with localized illness reported less open communication than those with advanced illness; open communication between couples with recurrent and advanced illnesses were similar. Patterns of change in communication over time did not vary by phase of illness.
Aim 2
Certain time-varying psychosocial and cancer-related factors affected perceived dyadic communication over time. When facing the same phase of illness at the same time point of survivorship, couples’ open communication increased as their social support increased, uncertainty decreased, and hormonal symptoms in patients reduced. Couples’ demographics, length of relationship, patients’ prostate cancer-specific bowel, sexual, and urinary symptoms, and couples’ general symptoms did not affect their open communication.
DISCUSSION
This study used multilevel modeling to examine patterns of change in the levels of dyadic open communication over time among couples managing prostate cancer. The results corroborate findings of previous research using qualitative or cross-sectional retrospective approaches: communication about cancer-related issues between prostate cancer patients and spouses decreased over time [7, 24, 28, 61]. At diagnosis, patients and partners need to deal with imminent illness-related information, which may motivate them to communicate more openly to adjust to their new reality, to make decisions, and to address family relationship concerns. Upon the completion of treatment, couples strive to return to normal life, and thus, may push concerns about long-term side-effects and prognosis to the background and rarely address them [24]. As couples gradually switch focus away from cancer to other parts of their lives, their needs for sharing cancer-related issues may decrease. Some couples may also deliberately avoid talking about the cancer situation, especially the emotions, as a way of avoiding preoccupation with the illness [24].
Prior research helps to explain the overall downward trend in communication for both partners in this study. Qualitative research has found that couples with prostate cancer in general, but particularly men, have a strong desire to get their lives back together and move beyond the illness, which may limit their open expression of fears and feelings [33]. Some research suggests that, as men withdraw, they appreciate it when their wives respect their needs for retreat [24]. In response to patients’ reticence, the partners reciprocally reduce communication even though they may want to talk more [33]. As one person persistently holds back cancer-related information, it may in turn make the other partner give up the effort to communicate [62].
In this study, the phase of illness predicted dyadic open communication at diagnosis but not patterns of change in communication over time. These findings indicate that couples’ open communication at diagnosis may be affected more by the phase of illness, whereas its patterns of change over time are affected more by their role of being a patient or spouse. Compared to advanced cancer, localized prostate cancer is considered a “good cancer” that can be cured [61]. Furthermore, patients with localized cancer usually have fewer or less severe symptoms and better QOL [42, 63]. Couples in the localized phase, thus, may not have urgent needs to communicate at diagnosis. In contrast, advanced cancers are associated with more ongoing physical and psychosocial threats and disturbances [18, 42, 64], which increase couples’ need to share information, feelings, and thoughts that are related to imminent treatment decisions and caregiving needs.
This study also provided evidence that certain psychosocial factors affected dyadic communication. Greater support was related to more open communication. Social support plays an important role in adaptation during survivorship [65-67]. It is possible that the information and tangible support from their social network help normalize couples’ feelings and reduce their vulnerability. Couples, thus, may engage in more open communication with each other, especially about those private feelings and thoughts that they do not want to share with others. Wives usually have larger support networks than prostate cancer patients [33]. These networks help the adjustment of the well partner [66], which make her more available and resourceful to the patient when he shares concerns and feelings.
The finding that perceived dyadic communication decreased as couples’ uncertainty increased is counter-intuitive. Couples often experience significant uncertainty related to the symptoms, treatment, and prognosis when managing prostate cancer [24, 39, 61, 68-69]. Our results suggest that the lack of knowledge about the situation and/or the effects of the situation can make patients and partners unsure of how to initiate a discussion about the illness [24] or about what to say to one another that is helpful [24, 33]. Couples, thus, may avoid communication for fear of distressing themselves or each other. Mishel has indicated that uncertainty reduces a person’s sense of personal resources to manage the situation [70-71]. Our findings further suggest that uncertainty decreases not only personal resources as noted by Mishel, but also reduces couples’ interpersonal resources, i.e., their ability to communicate with one another about the illness.
Another clinically relevant finding was that reduced hormonal symptoms in men (e.g., hot flashes and breast tenderness) affected dyadic open communication. Previous qualitative research found that men not only had difficulties adjusting to the physical changes but were uncomfortable disclosing feelings about these changes to wives [33]. Women, in respect for men’s fragility and/or for fear of creating problems may not have asked about how the patient is doing [24, 33]. Couples generally believe that there is no use spending time thinking or talking about symptoms. They prefer to deal with the problems when they arise [24]. Engaging in the “don’t talk, don’t ask” strategy allows patients and partners to believe they are protecting themselves and their loved ones by downplaying discouragement or embarrassment associated with certain symptoms. Yet, concealing symptoms decreases couples’ understanding of disease and treatment outcomes and makes partners less likely to obtain support from each other.
Unlike previous research in other types of cancer [7, 28], we did not find significant relationships between couple’s demographics and their open communication. This may be due to the homogeneity of participants in race, education, and length of relationship. Future research needs to include participants with more diverse socio-demographic backgrounds.
Finally, this study has provided empirical support for the assumption that communication is a multidimensional, interactional process [72]. Our results show that the total variability in dyadic open communication was partitioned across intra-personal, intra-couple, and inter-couple levels, indicating that couples’ communication is affected by variables at personal and couple levels. The effects of time-varying factors also confirm that communication is affected by the context of survivorship. Thus, future studies need to consider the social contextual factors (e.g., social support) at different levels during different phases of survivorship when tackling the complexity of interpersonal communication.
The Benefits of Using Multilevel Modeling
One major contribution of this study is the use of multilevel modeling to improve the statistical analysis techniques available to communication researchers. Communication is a complex and interactive process between people, and thus, more advanced statistical methods are needed to detect the variances at different levels in quantitative research, especially when handling longitudinal data. Some of our findings might otherwise have gone undetected if traditional methods were used [59, 73]. Using multilevel modeling also allowed the estimation of variance in the patterns of changes in couple’ open communication across time as a function of time-varying variables.
Limitations
Although the analyses yielded important findings, this study has following limitations. First, the sample consisted of primarily Caucasian and African American, well-educated couples with a long relationship history. Participants with more diverse racial and socio-demographic backgrounds are needed to obtain an in-depth understanding on how social and cultural contexts affect the patterns of partners’ interactions during survivorship. Second, the inclusion of all male patients and female partners limited the investigation of how gender affects dyadic open communication in the context of cancer. Lastly, the communication instrument we used measures each partner’s perception of their communication in general. It does not distinguish between each individual’s behaviors as the source versus the receiver of the information exchange process. Neither does the instrument examine other aspects of communication that may be of importance to couples’ survivorship (e.g., their need for cancer-related communication or their satisfaction with their communication).
Nonetheless, this study described patterns of perceived dyadic communication over time, using prospective data that were obtained at multiple time points. Our findings not only help understand couples’ interaction patterns during cancer survivorship but also provide evidence of factors to consider when designing appropriate interventions (e.g., promote social support and reduce uncertainty) to improve open communication between cancer patients and partners
Acknowledgments
The study in this report was funded in part by a research grant from National Cancer Institute (R01CA10738, PI: Northouse) and by an individual NRSA (F31NR010990, PI: Song). LS is currently sponsored by an institutional NRSA for postdoctoral training (5T32NR007091, PI: Mishel).
References
- 1.Lewis FM. The Family’s “Stuck Points” in Adjusting to Cancer. In: Holland JC, Breitbart WS, Jacobsen PB, et al., editors. Psycho-Oncology. Oxford University Press; New York: 2010. pp. 511–15. [Google Scholar]
- 2.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–18. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery J, Fewer W. Family Systems and Beyond. Human Science Press, INC; 1988. [Google Scholar]
- 4.Rogers LE, Escudero V, editors. Relational communication: an interactional perspective to the study of process and form. Mahwah, N.J.: Lawrence Erlbaun Associates; 2004. p. 248. [Google Scholar]
- 5.Song LX. Couples’ Communication and Quality of Life During Prostate Cancer Survivorship. University of Michigan; Ann Arbor: 2009. [Google Scholar]
- 6.Manne S, Ostroff J, Norton TR, et al. Cancer-related relationship communication in couples coping with early stage breast cancer. Psycho-Oncology. 2006;15:234–247. doi: 10.1002/pon.941. [DOI] [PubMed] [Google Scholar]
- 7.Porter LR, Keefe FJ, Hurwitz H, et al. Disclosure between patients with gastronintestinal cancer and their spouses. Psycho-Oncology. 2005;14:1030–1042. doi: 10.1002/pon.915. [DOI] [PubMed] [Google Scholar]
- 8.Langer SL, Brown JD, Syrjala KL. Intrapersonal and interpersonal consequences of protective buffering among cancer patients and caregivers. Cancer. 2009;115:4311–4325. doi: 10.1002/cncr.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manne SL, Norton TR, Ostroff JS, et al. Protective buffering and psychological distress among couples coping with breast cancer: The moderating role of relationship satisfaction. Journal of Family Psychology. 2007;21:380–388. doi: 10.1037/0893-3200.21.3.380. [DOI] [PubMed] [Google Scholar]
- 10.Manne S, Badr H, Zaider T, et al. Cancer-related communication, relationship intimacy, and psychological distress among couples coping with localized prostate cancer. J Cancer Surviv. 2010;4:74–85. doi: 10.1007/s11764-009-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradis M, Consoli SM, Pelicier N, et al. Psychosocial distress and communication about cancer in ill partners and their spouses. Encephale. 2009;35:146–51. doi: 10.1016/j.encep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Gacci M, Simonato A, Masieri L, et al. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health Qual Life Outcomes. 2009;7:94. doi: 10.1186/1477-7525-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. New England Journal of Medicine. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 14.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. Journal of Clinical Oncology. 2005;23:2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld B, Roth AJ, Gandhi S, et al. Differences in health-related quality of life of prostate cancer patients based on stage of cancer. Psycho-Oncology. 2004;13:800–7. doi: 10.1002/pon.797. [DOI] [PubMed] [Google Scholar]
- 16.Green HJ, Pakenham KI, Headley BC, et al. Coping and health-related quality of life in men with prostate cancer randomly assigned to hormonal medication or close monitoring. Psycho-Oncology. 2002;11:401–14. doi: 10.1002/pon.599. [DOI] [PubMed] [Google Scholar]
- 17.Steginga SK, Occhipinti S, Dunn J, et al. The supportive care needs of men with prostate cancer. Psycho-Oncology. 2001;10:66–75. doi: 10.1002/1099-1611(200101/02)10:1<66::aid-pon493>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Lintz K, Moynihan C, Steginga S, et al. Prostate cancer patients’ support and psychological care needs: Survey from a non-surgical oncology clinic. Psycho-Oncology. 2003;12:769–83. doi: 10.1002/pon.702. [DOI] [PubMed] [Google Scholar]
- 19.Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CaPSURE. Journal of Urology. 2004;171:703–7. doi: 10.1097/01.ju.0000107964.61300.f6. [DOI] [PubMed] [Google Scholar]
- 20.Davison BJ, So AI, Goldenberg SL. Quality of life, sexual function and decisional regret at 1 year after surgical treatment for localized prostate cancer. BJU International. 2007;100:780–5. doi: 10.1111/j.1464-410X.2007.07043.x. [DOI] [PubMed] [Google Scholar]
- 21.Harden JK, Northouse LL, Mood DW. Qualitative Analysis of Couples’ Experience With Prostate Cancer by Age Cohort. Cancer Nursing. 2006;29:367–377. doi: 10.1097/00002820-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Sunny L, Hopfgarten T, Adolfsson J, et al. Economic conditions and marriage quality of men with prostate cancer. BJU International. 2007;99:1391–7. doi: 10.1111/j.1464-410X.2007.06807.x. [DOI] [PubMed] [Google Scholar]
- 23.Krongrad A, Lai H, Burke MA, et al. Marriage and mortality in prostate cancer. Journal of Urology. 1996;156:1696–70. [PubMed] [Google Scholar]
- 24.Gray RE, Fitch M, Phillips C, et al. Managing the impact of illness: The experiences of men with prostate cancer and their spouses. Journal of Health Psychology. 2000;5:531. doi: 10.1177/135910530000500410. [DOI] [PubMed] [Google Scholar]
- 25.Lavery JF, Charke VA. Prostate Cancer: Patients’ and Spouses’ Coping and Marital Adjustment. Psychology, Health & Medicine. 1999;4:289–302. [Google Scholar]
- 26.Badr H, Taylor CLC. Sexual dysfunction and spousal communication in couples coping with prostate cancer. Psycho-Oncology. 2009;18:735–746. doi: 10.1002/pon.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson BN, Davison KP, Pennebaker JW, et al. Disease disclosure patterns among breast cancer patients. Psychology and Health. 2002;17:51–62. [Google Scholar]
- 28.Hilton BA. Family communication patterns in coping with early breast cancer. Western Journal of Nursing Research. 1994;16:366–88. doi: 10.1177/019394599401600403. [DOI] [PubMed] [Google Scholar]
- 29.American Cancer Society. Cancer Facts & Figures 2009. Atlanta: 2009. [Google Scholar]
- 30.American Cancer Society. Cancer Facts & Figures 2007. Atlanta: 2007. [Google Scholar]
- 31.SEER. Cancer Stat Fact Sheets. National Cancer Institute; 2006. [Google Scholar]
- 32.Steverink N, Westerhof GJ, Bode C, et al. The personal experience of aging, individual resources, and subjective well-being. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2001;56:P364–73. doi: 10.1093/geronb/56.6.p364. [DOI] [PubMed] [Google Scholar]
- 33.Boehmer U, Clark JA. Communication about prostate cancer between men and their wives. Journal of Family Practice. 2001;50:226–31. [PubMed] [Google Scholar]
- 34.Arrington MI, Grant CH, Vanderford ML. Man to man and side by side, they cope with prostate cancer: self-help and social support. Journal of Psychosocial Oncology. 2005;23:81–102. doi: 10.1300/j077v23n04_05. [DOI] [PubMed] [Google Scholar]
- 35.Arrington MI. “She’s right behind me all the way”: an analysis of prostate cancer narratives and changes in family relationships. Journal of Family Communication. 2005;5:141–62. [Google Scholar]
- 36.Boehmer U, Clark JA. Married couples’ perspectives on prostate cancer diagnosis and treatment decision-making. Psycho-Oncology. 2001;10:147–55. doi: 10.1002/pon.504. [DOI] [PubMed] [Google Scholar]
- 37.Fergus KD, Gray RE, Fitch MI, et al. Active consideration: conceptualizing patient-provided support for spouse caregivers in the context of prostate cancer. Qualitative Health Research. 2003;12:492–514. doi: 10.1177/104973202129120034. [DOI] [PubMed] [Google Scholar]
- 38.Gray RE, Fitch M, Phillips C, et al. To tell or not to tell: patterns of disclosure among men with prostate cancer. Psycho-Oncology. 2000;9:273–82. doi: 10.1002/1099-1611(200007/08)9:4<273::aid-pon463>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 39.Hawes S, Malcarne V, Ko C, et al. Identifying problems faced by spouses and partners of patients with prostate cancer. Oncology Nursing Forum Online. 2006;33:807–14. doi: 10.1188/06.ONF.807-814. [DOI] [PubMed] [Google Scholar]
- 40.Northouse LL, McCorkle R. Spouse caregivers of cancer patient. In: Holland JC, Breitbart WS, Jacobsen PB, et al., editors. Psycho-oncology. Oxford University Press, Inc; New York: 2010. pp. 516–21. [Google Scholar]
- 41.Lewis FM. Advancing Family Focused Oncology Nursing Research. In: Phillips JM, King CR, editors. Advancing Oncology Nursing Science. Oncology Nursing Society Publishing Division; Pittsburgh, Pennsylvania: 2009. pp. 409–35. [Google Scholar]
- 42.Northouse LL, Mood D, Montie JE, et al. Living with Prostate Cancer: Patients’ and Spouses’ Psychosocial Status and Quality of Life. Journal of Clinical Oncology. 2007;25:4171–4177. doi: 10.1200/JCO.2006.09.6503. [DOI] [PubMed] [Google Scholar]
- 43.Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology. 2002;20:4050–64. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 44.Northouse LL, Mood D, Templin T, et al. Couples’ patterns of adjustment to colon cancer. Social Science & Medicine. 2000;50:271–84. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 45.Ward S, Leventhal H, Easterling D, et al. Social support, self-esteem, and communication in patients receiving chemotherapy. Journal of Psychosocial Oncology. 1991;9(1):95–116. [Google Scholar]
- 46.Quartana PJ, Schmaus BJ, Zakowski SG. Gender, neuroticism, and emotional expressivity: effects on spousal constraints among individuals with cancer. Journal of Consulting & Clinical Psychology. 2005;73:769–76. doi: 10.1037/0022-006X.73.4.769. [DOI] [PubMed] [Google Scholar]
- 47.Pickles T, Ruether JD, Weir L, et al. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU International. 2007;100:544–51. doi: 10.1111/j.1464-410X.2007.06981.x. [DOI] [PubMed] [Google Scholar]
- 48.Mishel MH, Germino BB, Belyea M, et al. Moderators of an uncertainty management intervention: for men with localized prostate cancer. Nursing Research. 2003;52:89–97. doi: 10.1097/00006199-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Family Practice. 2003;20:724–9. doi: 10.1093/fampra/cmg617. [DOI] [PubMed] [Google Scholar]
- 50.Badr H, Carmack Taylor CL. Social constraints and spousal communication in lung cancer. Psycho-Oncology. 2006;15:673–83. doi: 10.1002/pon.996. [DOI] [PubMed] [Google Scholar]
- 51.Northouse LL, Rosset T, Phillips L, et al. Research with families facing cancer: The challenges of accrual and retention. Research in Nursing & Health. 2006;29:199–211. doi: 10.1002/nur.20128. [DOI] [PubMed] [Google Scholar]
- 52.Lewis FM. Family Home Visitation Study Final Report. National Cancer Institute, National Institutes of Health; Bethesda, MD: 1996. [Google Scholar]
- 53.Mood D, Song L, Kershaw T, et al. Assessing risk for distress in cancer patients and family caregivers. Oncology Nursing Forum 2007. 2007;34(1):233. [Google Scholar]
- 54.Brandt PA, Weinert C. The PRQ--a social support measure. Nursing Research. 1981;30:277–80. [PubMed] [Google Scholar]
- 55.Mishel M, Epstein D. Uncertainty in Illness Scales: Manual. University of Arizona; Tucson AZ: 1990. [Google Scholar]
- 56.Northouse L, Mood D, Kershaw T, et al. Effects of a family intervention on men with prostate cancer and their spouses. Oncology Nursing Forum. 2007;34:176–7. [Google Scholar]
- 57.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 58.Northouse LL, Caffey M, Deichelbohrer L, et al. The quality of life of African American women with breast cancer. Research in Nursing & Health. 1999;22:449–60. doi: 10.1002/1098-240x(199912)22:6<449::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 59.West BT, Welch KB, Galecki A. Linear Mixed Models: a practical guide using statistical software. Boca Raton, Florida: Chapman & Hall/CRC: Taylor & Francis Group; 2007. [Google Scholar]
- 60.SAS Institute Inc. SAS 9.2. SAS Institute Inc.; Cary, NC, USA: 2008. [Google Scholar]
- 61.Maliski SL, Heilemann MV, McCorkle R. From “death sentence” to “good cancer”: couples’ transformation of a prostate cancer diagnosis. Nursing Research. 2002;51:391–7. doi: 10.1097/00006199-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Zhang AY, Siminoff LA. Silence and cancer: why do families and patients fail to communicate? Health Communication. 2003;15:415–29. doi: 10.1207/S15327027HC1504_03. [DOI] [PubMed] [Google Scholar]
- 63.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–8. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 64.Navon L, Morag A. Advanced prostate cancer patients’ relationships with their spouses following hormonal therapy. European Journal of Oncology Nursing. 2003;7:73–80. doi: 10.1016/s1462-3889(03)00022-x. discussion 81-2. [DOI] [PubMed] [Google Scholar]
- 65.Schag CA, Ganz PA, Wing DS, et al. Quality of life in adult survivors of lung, colon and prostate cancer. Quality of Life Research. 1994;3:127–41. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 66.Kershaw T, Mood D, Newth G, et al. Longitudinal analysis of a model to predict quality of life in prostate cancer patients and their spouses. Annals of Behavioral Medicine. 2008;36:117–28. doi: 10.1007/s12160-008-9058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ptacek JT, Pierce GR, Ptacek JJ. The social context of coping with prostate cancer. Journal of Psychosocial Oncology. 2002;20:61. doi: 10.1300/J077v25n02_03. [DOI] [PubMed] [Google Scholar]
- 68.Kunkel EJS, Bakker JR, Myers RE, et al. Biopsychosocial Aspects of Prostate Cancer. Psychosomatics. 2000;41:85–94. doi: 10.1176/appi.psy.41.2.85. [DOI] [PubMed] [Google Scholar]
- 69.Gray RE, Fitch MI, Phillips C, et al. Presurgery experiences of prostate cancer patients and their spouses. Cancer Practice. 1999;7:130–5. doi: 10.1046/j.1523-5394.1999.07308.x. [DOI] [PubMed] [Google Scholar]
- 70.Mishel MH, Padilla G, Grant M, et al. Uncertainty in illness theory: a replication of the mediating effects of mastery and coping. Nursing Research. 1991;40:236–40. [PubMed] [Google Scholar]
- 71.Mishel MH, Sorenson DS. Uncertainty in gynecological cancer: a test of the mediating functions of mastery and coping. Nursing Research. 1991;40:167–71. [PubMed] [Google Scholar]
- 72.Galvin KM, Dickson FC, Marrow SR. Engaging Theories in Family Communication - Multiple Perspectives. Thousand Oaks, California: Sage Publications, Inc; 2006. Systems Theory: Patterns and (W)holes in Family Communication. [Google Scholar]
- 73.Raudenbush SW, Bryk AS. Advanced Quantitative Techniques in the social sciences series. Thousand Oaks, California: Sage Publications, Inc; 2002. Hierarchical Linear Models: applications and data analysis methods. [Google Scholar]


