Abstract
Protein- and cell-based therapies represent highly promising strategies for regenerative medicine, immunotherapy, and oncology. However, these therapies are significantly limited by delivery considerations, particularly in terms of protein stability and dosing kinetics as well as cell survival, engraftment and function. Hydrogels represent versatile and robust delivery vehicles for proteins and cells due to their high water content that retains protein biological activity, high cytocompatibility and minimal adverse host reactions, flexibility and tunability in terms of chemistry, structure, and polymerization format, ability to incorporate various biomolecules to convey biofunctionality, and opportunity for minimally invasive delivery as injectable carriers. This review highlights recent progress in the engineering of poly(ethylene glycol) (PEG) hydrogels cross-linked using maleimide reactive groups for protein and cell delivery.
The Need for Delivery Vehicles for Protein and Cell Delivery
Protein- and cell-based therapies represent revolutionary strategies in regenerative medicine, oncology, treatment of inflammatory disorders, and immunology1-3 (Table 1). These next-generation therapeutics offer significant advantages over small pharmacological compounds in terms of specificity, control, and functionality. However, several challenges limit the broad application of protein- and cell-based therapeutics (Table 2). In particular, delivery considerations pose significant challenges to efficient and effective implementation.
Table 1.
Representative protein- and cell-based therapies or clinical trials and associated delivery vehicles.
| Application | Protein | Cell | Delivery Vehicle |
|---|---|---|---|
| spinal fusion | BMP-2 (Medtronic) | collagen sponge | |
| myocardial infarct | bone marrow-derived stem cells (Amorcyte) MSC (Osiris) | Saline | |
| rheumatoid arthritis, Crohn's disease & other inflammatory disorders | adalimumab TNF-α antibody (Humira, Abbott) | Saline | |
| stroke | neural stem cells (ReNeuron) | Saline | |
| diabetes | porcine β cells (Living Cell Tech) | Alginate | |
| cartilage | autologous chondrocytes (Genzyme) | Collagen | |
| breast cancer | trastuzumab HER2 antagonist antibody (Herceptin, Genentech) | Saline | |
| diabetes | insulin lispro (Humalog, Eli Lilly) | Saline | |
| leg ulcer | fibroblasts/keratinocytes (Organogenesis) | cell-derived matrix | |
| critical limb ischemia | MSC (Stempeutics) | Saline |
Table 2.
Considerations for protein and cell therapeutics.
| Protein | Cell |
|---|---|
| selection of therapeutic due to complex underlying biology | autologous, allogenic donor cells, including ex vivo manipulations |
| delivery route & vehicle | delivery route & vehicle |
| bioactivity & stability | cell dose, survival, & engraftment |
| dosing & clearance kinetics | mechanism of action: paracrine/trophic support vs. direct functional support |
| host response | host response |
| manufacturing, including expression, purification, sterilization | manufacturing, including sterilization |
| regulatory aspects, safety & monitoring | regulatory aspects, safety & monitoring |
Proteins, such as growth and differentiation factors, antibodies, and cytokines, represent important therapeutics in regenerative medicine, oncology, and other targeted therapies. For instance, protein-based therapeutics can directly promote tissue growth as in nerve regeneration, provide enzymes to digest scar tissue, recruit osteoprogenitors and induce differentiation into bone-forming cells, or promote angiogenesis/vascularization to modulate tissue healing and repair. In cancer therapeutics, protein-based interventions can modulate new blood vessel growth at tumor sites and potentially ‘starve’ cancers of nutrients, or enable targeting of tumors based on surface biomarker expression on tumors. For protein-based therapeutics, important delivery considerations include delivery route, protein stability, and dosing kinetics (target dose, residence time). For example, vascular endothelial growth factor (VEGF) is a potent inducer of angiogenesis and vasculogenesis and has emerged has a promising therapeutic to treat conditions with reduced vascular perfusion, including peripheral artery disease, coronary heart disease and myocardial infarcts, and vascularization of tissue-engineered constructs4,5. However, injected VEGF is rapidly cleared from tissues, resulting in reduced therapeutic efficiency. Moreover, high local concentrations of VEGF induce a potent vascularization response but these vessels are often dysfunctional and leaky and regress once the exogenous growth factor is depleted. In contrast, delivery of VEGF from engineered carriers leads to sustained local levels of VEGF and functional vessels that persist beyond the residence time of the delivered factor6-8.
Cell-based therapies represent hugely promising therapeutic strategies in numerous areas of regenerative medicine and immunology. In particular, stem cell transplantation promotes therapeutic improvements in various deficiencies by providing cells that either engraft and differentiate into functional tissue constituents or secrete bioactive factors supporting host cellular activities. For example, delivery of mesenchymal stem cells and cardiomyocyte progenitors to restore cardiac function, either by secreting paracrine factors to recruit and enhance survival of endogenous cells or differentiation into contractile cells, is a promising strategy to treat myocardial infarcts9-11. Similarly, implantation of metabolically functional cells may provide long-term corrective function of metabolic deficiencies in ways that are superior to pharmacologic intervention. For instance, implantation of metabolically responsive, insulin-secreting cells has the potential to provide significantly better glycemic regulation than blood glucose monitoring and insulin injections12. Nevertheless, roadblocks to efficient cell delivery in terms of cell survival, engraftment, differentiation, and monitoring severely limit the widespread success and application of these strategies. Long term cell engraftment has been shown to correlate with enhanced therapeutic outcomes13-15, and has also been shown to be greater in cases in which cells are delivered in an appropriate biomaterial carrier16. For example, bone marrow stromal cells directly injected into myocardial infarcts have poor engraftment rates (<1%) and at best modest improvements in function17,9. Similarly, progressive loss of transplanted pancreatic islets due to poor vascularization and engraftment significantly limit the long-term success of this promising cell therapy for type 1 diabetes18,19. Therefore, new classes of cell delivery vehicles that promote cell survival, engraftment, and function, including integration with host cells and tissues, are necessary to fully realize the potential of cell therapy.
Biomaterial Delivery Vehicles
Countless materials have been explored as delivery vehicles for proteins and cells. General requirements for these delivery vehicles are enumerated in Table 3, but the importance and relevance of each requirement are strongly dependent on the target application. Materials derived from natural sources, such as collagen, hyaluronic acid, alginate, and chitosan, have been extensively used in regenerative medicine and tissue engineering as protein and cell delivery vehicles. These natural materials can be biologically active, promote cell adhesion and growth, display high cytocompatibility and acceptable inflammatory profiles, and can be enzymatically or hydrolytically degraded. However, natural materials are difficult to process and manufacture into formulations with target mechanical and biochemical properties, display lot-to-lot variability, and may carry some risk of immunogenicity and pathogen transmission. Consequently, synthetic materials, such as metals, ceramics and glasses, polymers, and composites, offer significant advantages over natural materials in terms of defined composition, control over mechanical and chemical properties, manufacturability and processing. Limitations of synthetic materials include lack of bio-functionality/bio-specificity, reduced cytocompatibility compared to natural materials, and uncontrolled inflammatory host responses that often lead to foreign body reaction and fibrosis, although recent synthetic materials have been functionalized with bioactive components (e.g, peptides) to diminish or overcome these limitations. In particular, polymers provide highly tailorable synthetic materials for cell and protein delivery applications. Polymers have been extensively used as controlled delivery vehicles for proteins in various configurations, including membranes for delivery from reservoirs, biodegradable matrices that release proteins as the polymer degrades in aqueous environments, micro- and nanoparticles and micelles, and mesh networks such as hydrogels20-23. Polymeric systems have also advantages as cell delivery vehicles as these can be formulated as injectable carriers, tailored to degrade at specified rates to promote replacement by repair tissue, functionalized with bioactive agents to direct cellular activities, and engineered to provide structural and biological support for cells24,25. Polymeric cell carriers come in diverse structural configurations including space-filling cross-linked and self-assembled networks, porous foams, micro- and nanofibrillar scaffolds, and scaffolds generated by 3-D printing and associated additive manufacturing technologies.
Table 3.
General requirements for protein and cell delivery vehicles.
| Protein Vehicle | Cell Carrier |
|---|---|
| delivery route | delivery format (e.g., injectable vs. pre-formed) |
| protein loading capacity | vehicle structural, mechanical, biochemical properties to support target cell activities |
| protein bioactivity & stability after encapsulation | immunoisolation considerations |
| release mechanisms & kinetics to match desired pharmacokinetics | cell loading & cytocompatibility |
| host response to protein & vehicle | host response & integration, including vehicle degradation to allow tissue ingrowth |
| vehicle residence time & clearance | Vascularization |
| manufacturing, including sterilization | manufacturing, including sterilization |
| regulatory aspects, safety & monitoring | regulatory aspects, safety & monitoring |
PEG-maleimide Hydrogel as Delivery Vehicles
An attractive class of materials for protein and cell delivery is hydrogels. Hydrogels are water-swollen physically or chemically cross-linked polymer networks that can be engineered from natural materials such as alginate and collagen or synthetic polymers such as polyethylene glycol (PEG) (Figure 1). Advantageous characteristics of hydrogels include retention of protein biological activity, high cytocompatibility and minimal adverse host reactions due to their high water content, flexibility and tunability in terms of chemistry, structure, and polymerization format, ability to incorporate various biomolecules to convey biofunctionality, and opportunity for minimally invasive delivery as injectable carriers. Excellent reviews on hydrogels for protein and cell delivery can be found elsewhere26-32.
Figure 1.
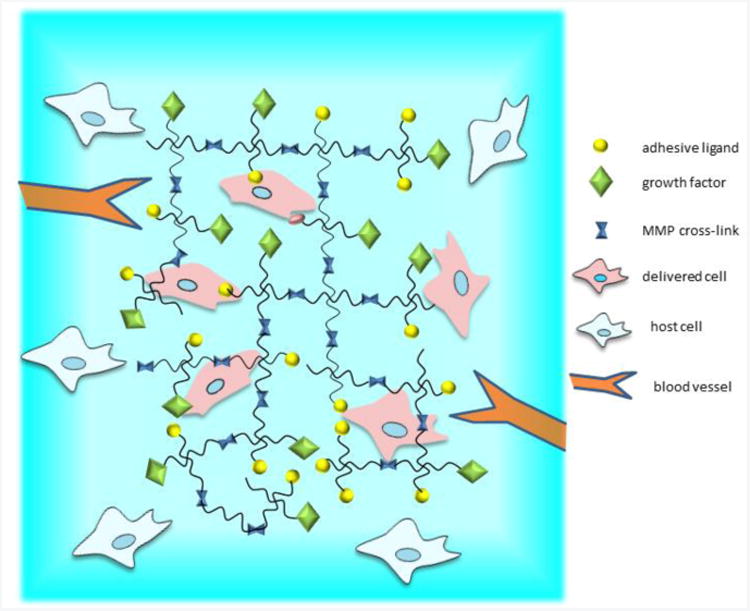
Biofunctional hydrogels for protein and cell delivery. Hydrogel network is functionalized with bioactive molecules, including cell adhesive peptides, protease-degradable cross-links, and growth factors. Hydrogel is designed to promote host cell interactions and blood vessel ingrowth to promote integration of transplanted donor cells.
PEG synthetic hydrogels represent the ‘gold standard’ in this field due to their intrinsic low-protein adsorption properties, minimal inflammatory profile and history of safe in vivo use, ease in incorporating various functionalities, and commercial availability of reagents such as macromers functionalized with different reactive end groups. Various cross-linking chemistries have been pursued to create biofunctionalized hydrogel networks of PEG macromers, with Michael-type addition reactions and acrylate polymerization being the most widely used30,33. Cross-linking chemistry, gelation time, polymer network structure (mesh size), swelling, and degradation properties are important considerations when selecting a hydrogel for protein- and cell-delivery applications. In PEG-diacrylate hydrogels, macromers are cross-linked via free-radical polymerization of acrylate end groups. Free radicals are created either by chemical activation or UV cleavage of a photoinitiator with the added ability to spatially control incorporation of bioligands or mechanical properties through additive or subtractive photo-patterning34,35. Free-radical polymerization cross-linking, however, is limited by cytotoxicity (especially in the case of sensitive cells such as pancreatic islets and neurons), non-ideal network structure containing poly(acrylate) chains of various sizes, and challenges related to in situ photo cross-linking for in vivo delivery applications. In contrast, for hydrogels cross-linked by Michael-type addition, functionalized end groups on branched PEG macromers are reacted with bi-functional or branched cross-linking molecules. Michael-addition PEG hydrogels based on 4-or 8-arm PEG macromers with acrylate, vinyl-sulfone, and thiol end-groups have been extensively investigated36-47. Michael-type addition cross-linking avoids the use of cytotoxic free-radicals and UV light, but instead require a nucleophilic reagent, such as triethanolamine (TEA), to facilitate the addition reaction. However, hydrogels formed in the presence of high concentrations of TEA have cytotoxic effects on sensitive cell types such as endothelial cells, ovarian follicular cells, and pancreatic islets48,49.
We have recently established maleimide groups as an alternative cross-linking chemistry for PEG hydrogels50. The maleimide reactive group is extensively used in peptide bioconjugate chemistry because of its fast reaction kinetics and high specificity for thiols at physiological pH. Maleimide-based cross-linking has significant advantages over other cross-linking chemistries, namely well-defined hydrogel structure, stoichiometric incorporation of bioligands, increased cytocompatibility, improved cross-linking efficiency, and reaction time scales appropriate for in situ gelation for in vivo applications50. Additionally, the base macromer exhibits minimal toxicity and inflammation in vivo and is rapidly excreted via the urine51 – important considerations in establishing the safety and translational potential of these hydrogels. We next present two examples of applications of PEG-maleimide hydrogels for protein and cell delivery.
PEG Hydrogel-based Delivery of Therapeutic Proteins for Cardiac Repair
Acute myocardial infarction caused by ischemia and reperfusion is the most common cause of cardiac dysfunction due to local cell death and inflammatory responses and fibrosis52,53. Protein therapeutics targeting different elements of the infarct cascade are being explored to enhance endogenous cell survival, modulate inflammation, reduce fibrosis, and promote repair54-56. However, direct protein injection into the myocardium has proven inefficient as the therapeutic proteins are rapidly cleared, thereby limiting the effective tissue dose. To address this delivery limitation, synthetic and natural hydrogels have been developed for controlled delivery of proteins and cells57-68. These therapeutic vehicles promote myocardial function and repair by supporting endogenous and transplanted cell survival and/or recruiting endogenous progenitor cells. Additionally, evidence is accumulating that natural hydrogels consisting of hyaluronic acid or decellularized ventricular extracellular matrix without exogenous therapeutic factors reduce infarct expansion and negative post-infarct remodeling possibly by providing mechanical support69-72. These biomaterial strategies are discussed in an excellent review73.
We engineered hydrogels for protein delivery in order to harness endogenous cell repair to enhance myocardial repair and function74. PEG-maleimide hydrogels cross-linked with a protease-degradable peptide were loaded with hepatocyte and vascular endothelial growth factors (HGF, VEGF) and delivered to the infarcted myocardium of rats. The hydrogel mesh size is on the order of 35-50 nm and provides a barrier for the release of HGF and VEGF, but in the presence of proteases, the peptide cross-linkers are degraded, resulting in sustained release of HGF and VEGF. The released protein maintains equivalent bioactivity as soluble protein51, demonstrating that this delivery vehicle supports the stability of encapsulated proteins. When delivered to the border zones following ischemia-reperfusion injury, there was no acute effect on cardiac function as measured by echocardiography. However, there was a time-dependent increase in angiogenesis, c-kit-positive stem cell recruitment, and decrease in fibrosis in infarcts treated with hydrogel co-delivering VEGF and HGF compared to direct injection of these proteins, hydrogels delivering single proteins, empty hydrogels, and untreated injured controls (Figure 2). Importantly, the dual growth factor-delivering hydrogel led to improvements in chronic cardiac function as measured by both invasive hemodynamics and echocardiography (Figure 2). These results demonstrate that dual growth factor release of HGF and VEGF from a bioactive hydrogel has the capacity to significantly improve cardiac remodeling and function following ischemia-reperfusion injury.
Figure 2.
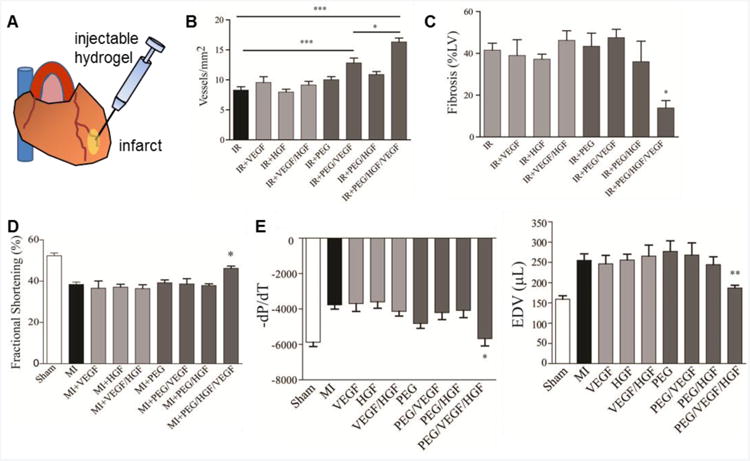
PEG-maleimide hydrogels for protein delivery to myocardial infarcts. (A) Hydrogels cross-linked with a protease-degradable peptide and loaded with HGF and VEGF were injected into the infarcted myocardium. Infarcts treated with hydrogel co-delivering VEGF and HGF (B) enhanced angiogenesis (*p<0.05, ***p<0.001) and (C) decreased fibrosis (*p<0.05) at 21 days post-treatment compared to direct injection of these proteins, hydrogels delivering single proteins, empty hydrogels, and untreated injured controls. Dual growth factor-delivering hydrogel led to improvements in (D) fractional shortening (*p<0.05) and (E) hemodynamics (dP/dT = change in pressure over time, EDV = end-diastolic volume, *p<0.05, **p<0.01) at 21 days post-treatment. Adapted from Ref. 74.
PEG-maleimide Hydrogels for Pancreatic Islet Delivery and Engraftment
Type 1 diabetes (T1DM) affects one in every 400 children and adolescents in the US75. Standard therapy with exogenous insulin is burdensome, associated with a significant danger of hypoglycemia, and only partially efficacious in preventing long term complications. Pancreatic islet transplantation has emerged as a promising therapy for T1DM76,77. Despite impressive initial improvements in metabolic control, few islet transplant patients maintain long term insulin independence12,78. Moreover, islet transplantation therapy is limited by inadequate supply of donor islets, a problem worsened by islet loss post-transplantation. Instant blood-mediated inflammatory reaction and toxic responses to immunosuppressive drugs contribute to progressive islet loss. Furthermore, inadequate vascularization of transplanted islets remains a significant cause of reduced islet viability, function, and engraftment79-82,77. Therefore, there is clear need for islet delivery vehicles that promote islet survival, vascularization, and function.
Biomaterial strategies for islet transplantation (reviewed in83,84) have centered on (i) semi-permeable barriers for encapsulation and immunoprotection85-90 and (ii) delivery vehicles for factors that support islet survival and/or vascularization91-97. We recently engineered an injectable vasculogenic, PEG-maleimide hydrogel to enhance the survival, vascularization, and engraftment of transplanted pancreatic islets in a mouse model of T1DM51 (Figure 3). VEGF, a potent stimulator of angiogenesis, was incorporated into the hydrogel and released in an on-demand manner through protease-mediated degradation of the hydrogel network (Figure 3). The PEG-maleimide hydrogel exhibited extended in vivo release of VEGF compared to other carriers such as alginate49. Isolated islets encapsulated in PEG- maleimide hydrogels displayed enhanced viability and insulin secretion compared to islets encapsulated in other hydrogels, including PEG-diacrylate and collagen I49. This injectable hydrogel was then used to deliver islets to the small bowel mesentery, a metabolically relevant site for insulin release, in diabetic mice. Controlled presentation of VEGF and RGD cell adhesive peptides within this hydrogel significantly improved the vascularization and function of transplanted islets. Diabetic mice receiving islets transplanted in proteolytically degradable hydrogels incorporating VEGF exhibited complete reversal of diabetic hyperglycemia with a 40% reduction in the number of islets required to achieve normoglycemia51 (Figure 3). Furthermore, hydrogel-delivered islets significantly improved weight gain, regulation of a glucose challenge, and intra-islet vascularization and engraftment compared to the clinical standard of islet infusion through the hepatic portal vein (Figure 3). This study establishes a simple biomaterial strategy for islet transplantation to enhance islet engraftment and function.
Figure 3.
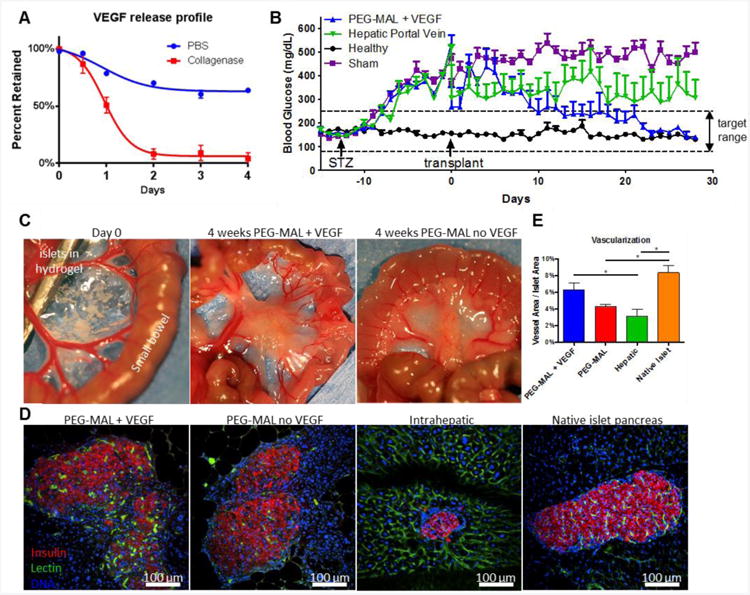
Vasculogenic PEG-maleimide hydrogels for pancreatic islet cell engraftment and function. (A) VEGF release profile from collagenase-degrading gels or gels treated in PBS as measured by ELISA showing on-demand release of VEGF. (B) Random daily blood sugar levels in streptozotocin-induced diabetic mice transplanted within syngeneic islets (400 islets). Only islets delivered within PEG-maleimide hydrogels with VEGF restored normoglycemia (p<0.001). (C) Transplant site in the small bowel mesentery at day 0 and at 4 weeks demonstrating significant remodeling of the PEG-maleimide hydrogel. (D) Islet graft explants (4 weeks) with patent vascular structures stained with IV-perfused FITC-lectin (green), DAPI (blue), and immunostained for insulin (red). (E) Quantification of vascular area normalized to islet area p<0.05). Adapted from Ref. 51.
Conclusions and Outlook
Promising protein- and cell-based therapies are significantly limited by delivery considerations, particularly in terms of protein stability and dosing kinetics as well as cell survival, engraftment and function. Hydrogels represent versatile and robust delivery vehicles for proteins and cells due to the retention of protein biological activity, high cytocompatibility and minimal adverse host reactions, flexibility and tunability in terms of chemistry, structure, and polymerization format, ability to incorporate various biomolecules to convey biofunctionality, and opportunity for minimally invasive delivery as injectable carriers. Biofunctional hydrogels have shown promise in pre-clinical models for diverse regenerative medicine applications but safety and functional data in rigorous animal models are necessary to establish the translational potential of these engineering materials. Among PEG-based hydrogels, maleimide-based cross-linked hydrogels offer significant advantages over other cross-linking chemistries, including well-defined hydrogel structure, stoichiometric incorporation of bioligands, increased cytocompatibility, improved cross-linking efficiency, and reaction time scales appropriate for in situ gelation for in vivo applications.
Continued progress in material design and synthesis strategies, including the application of orthogonal chemistries and novel macromolecular materials, will accelerate the development of tailorable, multi-functional delivery vehicles. Additionally, the engineering of stimulus-triggered functionalities for spatiotemporal control of mechanical properties, degradation and protein release kinetics, and cell-instructive activities will yield materials that better mimic tissues and promote the integration of transplanted or recruited endogenous cells with host tissues. Advances in immunology and stem cell biology will lead to the identification of potent biomolecules, such as immunoregulatory cytokines and cell recruitment factors, which can be integrated into delivery vehicles to generate immunomodulatory and reparative materials to harness endogenous repair. Finally, the combination of advanced imaging modalities for in vivo tracking of delivered proteins and cells and powerful transgenic animal models will provide rigorous platforms to evaluate engineered delivery vehicles.
Acknowledgments
AJG is partially supported by the NIH (R01 AR062368, R01 AR062920, DP3 DK094346), NSF (CBET 0939511) and the Juvenile Diabetes Research Foundation (17-2013-277).
Footnotes
Conflict of Interest Statement: No conflicts of interest
References
- 1.Leader B, Baca QJ, Golan DE. Protein therapeutics: A summary and pharmacological classification. Nature reviews. Drug discovery. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 2.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Science translational medicine. 2012;4:160sr4. doi: 10.1126/scitranslmed.3002717. [DOI] [PubMed] [Google Scholar]
- 3.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem cells translational medicine. 2012;1:51–8. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N. Vascular endothelial growth factor. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:789–91. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of vegf from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, Landazuri N, Thule PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107:3323–8. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized vegf-mimetic peptide. Biomaterials. 2011;32:5782–9. doi: 10.1016/j.biomaterials.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 9.Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clinical pharmacology and therapeutics. 2011;90:532–41. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Wang L, Jiang J, Zhou C, Guo T, Zheng S, Wang T. Cardiac stem cells and their roles in myocardial infarction. Stem cell reviews. 2012 doi: 10.1007/s12015-012-9421-4. [DOI] [PubMed] [Google Scholar]
- 11.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 13.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. Journal of the American College of Cardiology. 2009;54:1619–26. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marban E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circulation research. 2010;106:1570–81. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stabenfeldt SE, Munglani G, Garcia AJ, LaPlaca MC. Biomimetic microenvironment modulates neural stem cell survival, migration, and differentiation. Tissue Eng Part A. 2010;16:3747–58. doi: 10.1089/ten.tea.2009.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation. 2005;112:I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 18.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 19.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O'Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes care. 2012;35:1436–45. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark Saltzman W, Baldwin SP. Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev. 1998;33:71–86. doi: 10.1016/s0169-409x(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 21.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 22.Mehta M, Schmidt-Bleek K, Duda GN, Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012;64:1257–76. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Advanced healthcare materials. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 24.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 25.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annual Review of Biomedical Engineering. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials. 2006;18:1345–60. [Google Scholar]
- 29.Schmidt JJ, Rowley J, Kong HJ. Hydrogels used for cell-based drug delivery. J Biomed Mater Res A. 2008;87:1113–22. doi: 10.1002/jbm.a.32287. [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Anseth KS. Peg hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–43. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopecek J, Yang J. Smart self-assembled hybrid hydrogel biomaterials. Angew Chem Int Ed Engl. 2012;51:7396–417. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Advanced Materials. 18:2679–+. 2006. [Google Scholar]
- 35.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nature protocols. 2010;5:1867–87. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbert DL, Hubbell JA. Conjugate addition reactions combined with free-radical cross-linking for the design of materials for tissue engineering. Biomacromolecules. 2001;2:430–41. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 37.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. Mmp-2 sensitive, vegf-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A. 2004;68:704–16. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 38.Rizzi SC, Hubbell JA. Recombinant protein-co-peg networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part i: Development and physicochemical characteristics. Biomacromolecules. 2005;6:1226–38. doi: 10.1021/bm049614c. [DOI] [PubMed] [Google Scholar]
- 39.Rizzi SC, Ehrbar M, Halstenberg S, Raeber GP, Schmoekel HG, Hagenmuller H, Muller R, Weber FE, Hubbell JA. Recombinant protein-co-peg networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part ii: Biofunctional characteristics. Biomacromolecules. 2006;7:3019–29. doi: 10.1021/bm060504a. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Feng Zg, Zhang Ay, Sun Lg, Qian L. Synthesis and characterization of three-dimensional crosslinked networks based on self-assemly of α-cyclodextrins with thiolated 4-arm peg using a three-step oxidation. Soft Matter. 2006;2:343. doi: 10.1039/b517206c. [DOI] [PubMed] [Google Scholar]
- 41.Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Novel in situ forming, degradable dextran hydrogels by michael addition chemistry: Synthesis, rheology, degradation. Macromolecules. 2007;40:1165–73. [Google Scholar]
- 42.Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Rapidly in situ-forming degradable hydrogels from dextran thiols through michael addition. Biomacromolecules. 2007;8:1548–56. doi: 10.1021/bm061191m. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Wacker BK, Elbert DL. Thin polymer layers formed using multiarm poly(ethylene glycol) vinylsulfone by a covalent layer-by-layer method. Biomacromolecules. 2007;8:3682–6. doi: 10.1021/bm700756z. [DOI] [PubMed] [Google Scholar]
- 44.Chung IM, Enemchukwu NO, Khaja SD, Murthy N, Mantalaris A, Garcia AJ. Bioadhesive hydrogel microenvironments to modulate epithelial morphogenesis. Biomaterials. 2008;29:2637–45. doi: 10.1016/j.biomaterials.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu BH, Su J, Messersmith PB. Hydrogels cross-linked by native chemical ligation. Biomacromolecules. 2009;10:2194–200. doi: 10.1021/bm900366e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su J, Hu BH, Lowe WL, Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31:308–14. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Y, Kao WJ. In situ forming poly(ethylene glycol)-based hydrogels via thiol-maleimide michael-type addition. Journal of biomedical materials research Part A. 2011 doi: 10.1002/jbm.a.33106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011;32:2524–31. doi: 10.1016/j.biomaterials.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phelps EA, Templeman KL, Thule PM, Garcia AJ. Engineered vegf-releasing peg-mal hydrogel for pancreatic islet vascularization. Drug Deliv Transl Res. 2013 doi: 10.1007/s13346-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, Garcia AJ. Maleimide cross-linked bioactive peg hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater. 2012;24:64–70. 2. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34:4602–11. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeda FQ, Snell RJ, Parrillo JE. The contemporary management of acute myocardial infarction. Critical care clinics. 2001;17:411–34. doi: 10.1016/s0749-0704(05)70175-5. [DOI] [PubMed] [Google Scholar]
- 53.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (igf-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engel FB, Hsieh PC, Lee RT, Keating MT. Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:15546–51. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of pdgf-bb for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsur-Gang O, Ruvinov E, Landa N, Holbova R, Feinberg MS, Leor J, Cohen S. The effects of peptide-based modification of alginate on left ventricular remodeling and function after myocardial infarction. Biomaterials. 2009;30:189–95. doi: 10.1016/j.biomaterials.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 58.Yu J, Gu Y, Du KT, Mihardja S, Sievers RE, Lee RJ. The effect of injected rgd modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30:751–6. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 59.Li XY, Wang T, Jiang XJ, Lin T, Wu DQ, Zhang XZ, Okello E, Xu HX, Yuan MJ. Injectable hydrogel helps bone marrow-derived mononuclear cells restore infarcted myocardium. Cardiology. 2010;115:194–9. doi: 10.1159/000281840. [DOI] [PubMed] [Google Scholar]
- 60.Wall ST, Yeh CC, Tu RY, Mann MJ, Healy KE. Biomimetic matrices for myocardial stabilization and stem cell transplantation. J Biomed Mater Res A. 2010;95:1055–66. doi: 10.1002/jbm.a.32904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cittadini A, Monti MG, Petrillo V, Esposito G, Imparato G, Luciani A, Urciuolo F, Bobbio E, Natale CF, Sacca L, Netti PA. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail. 2011;13:1264–74. doi: 10.1093/eurjhf/hfr143. [DOI] [PubMed] [Google Scholar]
- 62.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a ph-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–16. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu LL, Reis LA, Momen A, Radisic M. Controlled release of thymosin beta4 from injected collagen-chitosan hydrogels promotes angiogenesis and prevents tissue loss after myocardial infarction. Regenerative medicine. 2012;7:523–33. doi: 10.2217/rme.12.35. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z, Wang H, Wang Y, Lin Q, Yao A, Cao F, Li D, Zhou J, Duan C, Du Z, Wang C. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials. 2012;33:3093–106. doi: 10.1016/j.biomaterials.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 65.Mathieu E, Lamirault G, Toquet C, Lhommet P, Rederstorff E, Sourice S, Biteau K, Hulin P, Forest V, Weiss P, Guicheux J, Lemarchand P. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PLoS One. 2012;7:e51991. doi: 10.1371/journal.pone.0051991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, Werner C. Sustained delivery of sdf-1alpha from heparin-based hydrogels to attract circulating pro-angiogenic cells. Biomaterials. 2012;33:4792–800. doi: 10.1016/j.biomaterials.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 67.Hunt NC, Shelton RM, Henderson DJ, Grover LM. Calcium-alginate hydrogel-encapsulated fibroblasts provide sustained release of vascular endothelial growth factor. Tissue Eng Part A. 2013;19:905–14. doi: 10.1089/ten.tea.2012.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith RR, Marban E, Marban L. Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel. Biomatter. 3:2013. doi: 10.4161/biom.24490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, Gorman JH, 3rd, Gorman RC, Burdick JA. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011;12:4127–35. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, Demaria AN, Dib N, Christman KL. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science translational medicine. 5:173ra25, 2013. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert opinion on drug delivery. 2013;10:59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 74.Salimath AS, Phelps EA, Boopathy AV, Che PL, Brown M, Garcia AJ, Davis ME. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One. 2012;7:e50980. doi: 10.1371/journal.pone.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National diabetes fact sheet. Centers for Disease Control and Prevention. 2005 [Google Scholar]
- 76.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8:1990–7. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 77.Vaithilingam V, Sundaram G, Tuch BE. Islet cell transplantation. Curr Opin Organ Transplant. 2008;13:633–8. doi: 10.1097/MOT.0b013e328317a48b. [DOI] [PubMed] [Google Scholar]
- 78.Alejandro R, Barton FB, Hering BJ, Wease S. 2008 update from the collaborative islet transplant registry. Transplantation. 2008;86:1783–8. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 79.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 80.Lakey JR, Mirbolooki M, Shapiro AM. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 81.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144:179–87. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16:1–8. doi: 10.3727/000000007783464461. [DOI] [PubMed] [Google Scholar]
- 83.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58:194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 84.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sawhney AS, Pathak CP, Hubbell JA. Modification of islet of langerhans surfaces with immunoprotective poly(ethylene glycol) coatings via interfacial photopolymerization. Biotechnol Bioeng. 1994;44:383–6. doi: 10.1002/bit.260440317. [DOI] [PubMed] [Google Scholar]
- 86.Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin. Bioconjug Chem. 2007;18:1713–5. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 87.Yun LD, Hee NJ, Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by pegylation and cyclosporine for 1 year. Biomaterials. 2007;28:1957–66. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 88.Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667–73. doi: 10.1016/j.matbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer peg hydrogels. Cell Transplant. 2008;16:1049–57. [PubMed] [Google Scholar]
- 90.Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin peg coating for intraportal islet transplantation. Nano Lett. 2008;8:1940–8. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, Bellocq JP, Pinget M, Kessler L. Influence of vegf on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12:627–35. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 92.Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, Mahato RI. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11:1105–16. doi: 10.1038/sj.gt.3302267. [DOI] [PubMed] [Google Scholar]
- 93.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, Kotb M, Gaber AO, Mahato RI. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21:15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Y, Liu YF, Zhang JL, Li TM, Zhao N. Elevation of vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World J Gastroenterol. 2007;13:2862–6. doi: 10.3748/wjg.v13.i20.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14:433–40. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 96.Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86:478–81. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brady AC, Martino MM, Pedraza E, Sukert S, Pileggi A, Camillo R, Hubbell J, Stabler CL. Pro-angiogenic hydrogels within macroporous scaffolds enhances islet engraftment in an extrahepatic site. Tissue Eng Part A. 2013 doi: 10.1089/ten.tea.2012.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]


