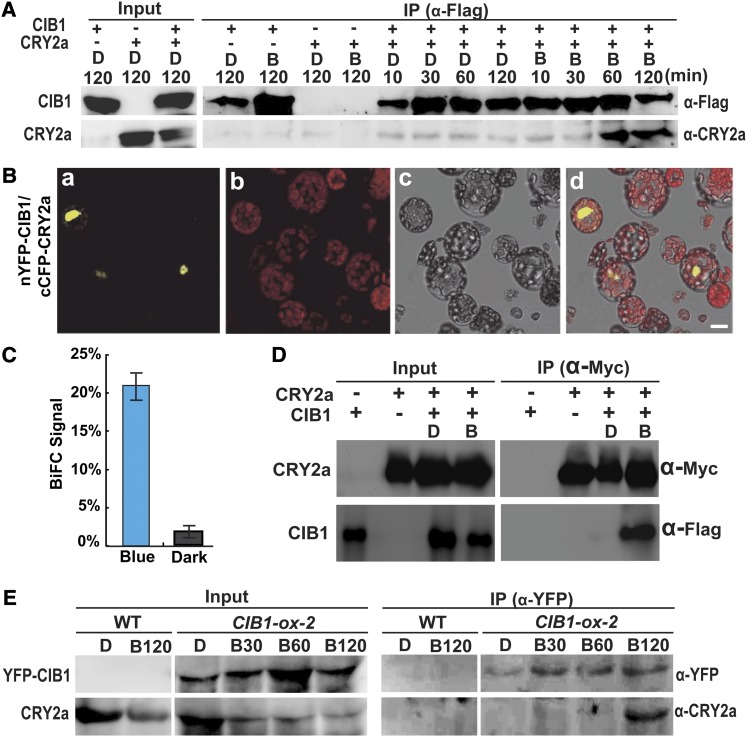
Figure 2.
CRY2a Interacts with CIB1 in Response to Blue Light in Vitro and in Plant Cells.
(A) A pull-down assay showing the blue light–dependent CRY2a–CIB1 interaction in vitro. Agarose beads conjugated with anti-Flag antibody (α-Flag) were mixed with the lysate of insect cells expressing 6His-CIB1-Flag (CIB1) and 6His-CRY2a (CRY2a). The mixture was treated with blue light (B, 22 μmol m−2 s−1) or darkness for the indicated durations. The bound proteins were eluted after washing and analyzed by immunoblots probed with anti-Flag antibody (α-Flag), stripped, and reprobed with anti-CRY2a antibody (α-CRY2a). IP, immunoprecipitation.
(B) BiFC assay showing the blue light–dependent CRY2a–CIB1 interaction in Arabidopsis protoplasts cotransfected with the plasmids encoding nYFP-CIB1 and cCFP-CRY2a. The mesophyll protoplasts of 4-week-old plants grown in LD (16 h light/8 h dark) conditions were cotransformed with plasmids encoding the indicated proteins, incubated for 12 h in the dark, and then transferred to blue light (22 μmol m−2 s−1) for 30 min prior to the confocal microscopy analysis. Image a, YFP fluorescence; image b, autofluorescence; image c, bright field; image d, merge of images a to c. Bar = 10 µm.
(C) The percentage of protoplasts that showed BiFC fluorescence signals was counted. Each sample contains at least 50 protoplasts. Means and sd (n = 3) are shown. P = 0.00026 (Student’s t test).
(D) Ex vivo coimmunoprecipitation assay showing blue light–dependent formation of the CRY2a-CIB1 complex in N. benthamiana. Young leaves were infiltrated with Agrobacteria harboring the plasmids encoding CIB1-Flag (CIB1) or CRY2a-Myc (CRY2a) as indicated, kept in continuous white light for 2 d, moved to darkness for 1 d, and then exposed to blue light (B; 22 μmol m−2 s−1) for 1 h or kept in darkness (D). The protein extracts were incubated with the agarose conjugated with anti-Myc antibody at 4°C for 60 min. Beads were collected and washed three times prior to the elution of immunoprecipitation products. Immunoblots of the total protein extracts (Input) and the IP product were performed using the anti-Myc antibody (α-Myc) and anti-Flag antibody (α-Flag), sequentially.
(E) Coimmunoprecipitation assays showing the blue light–dependent formation of the CRY2a-CIB1 complex in soybean. The wild-type (WT) soybean KN18 and a soybean transgenic line (line 2) overexpressing the Pro35S:YFP-CIB1 transgene (CIB1-ox-2) were grown in SD (8 h light/16 h dark) conditions for 2 weeks. Plants were transferred to darkness for 18 h and exposed to blue light (22 μmol m−2 s−1) for the time indicated periods of time (D, 0 min; B30, 30 min; B60, 60 min; B120, 120 min). Immunoblots of the protein extracts (Input) and the immunoprecipitation products using the agarose conjugated with anti-GFP antibody (α-GFP) were probed by anti-YFP antibody (α-YFP), stripped, and reprobed by anti-CRY2a antibody (α-CRY2a).