Rumex palustris and Rumex acetosa are two closely related species that survive flooding using distinct strategies. Using a genomics approach, this study identifies novel molecular components and processes that contribute to the survival of these plant species that normally complete their life cycle in flood-prone environments.
Abstract
Global climate change has increased flooding events, which affect both natural vegetation dynamics and crop productivity. The flooded environment is lethal for most plant species because it restricts gas exchange and induces an energy and carbon crisis. Flooding survival strategies have been studied in Oryza sativa, a cultivated monocot. However, our understanding of plant adaptation to natural flood-prone environments remains scant, even though wild plants represent a valuable resource of tolerance mechanisms that could be used to generate stress-tolerant crops. Here we identify mechanisms that mediate the distinct flooding survival strategies of two related wild dicot species: Rumex palustris and Rumex acetosa. Whole transcriptome sequencing and metabolite profiling reveal flooding-induced metabolic reprogramming specific to R. acetosa. By contrast, R. palustris uses the early flooding signal ethylene to increase survival by regulating shade avoidance and photomorphogenesis genes to outgrow submergence and by priming submerged plants for future low oxygen stress. These results provide molecular resolution of flooding survival strategies of two species occupying distinct hydrological niches. Learning how these contrasting flood adaptive strategies evolved in nature will be instrumental for the development of stress-tolerant crop varieties that deliver enhanced yields in a changing climate.
INTRODUCTION
Flooding is a globally occurring abiotic stress that has become increasingly frequent and unpredictable during the last decades. Flooding events, resulting in partial to complete inundation of plants, can have severe effects on the abundance and distribution of wild plant species in natural ecosystems (Silvertown et al., 1999; Voesenek et al., 2004) and on the productivity of crops (Voesenek and Bailey-Serres, 2009). An aqueous environment is stressful to terrestrial plants because of 104-fold slower rates of gas diffusion compared with air. The consequent limited exchange of gases such as CO2 and O2 dramatically limits photosynthesis and respiration, respectively. Furthermore, muddy and turbid floodwaters reduce light intensity, thereby further limiting photosynthesis. Ultimately, an imbalance between the production and consumption of carbohydrates coupled with an accumulation of toxic metabolic end products proves fatal for most nonadapted terrestrial plants (Colmer and Voesenek, 2009).
Some plants have evolved traits to avoid and ameliorate the problems associated with complete submergence. Rapid acceleration of shoot elongation growth enables some plant species to outgrow floodwaters and thus maintain fast gas exchange and re-establish aerial photosynthesis (Bailey-Serres and Voesenek, 2008). Such an escape strategy is energetically expensive because it requires considerable amounts of carbohydrates to fuel the rapid growth toward the water surface (Setter and Laureles, 1996). Therefore, escape growth is beneficial only if the flooding event is not too deep to outgrow and if the growth investment is rewarded by restored gas exchange and aerial photosynthesis as the leaves emerge above the water surface (Pierik et al., 2009). If the water surface is not reached, survival of escape-driven plants is severely reduced. Deep or transient flood conditions favor species with growth-suppressing behavior upon submergence by limiting carbohydrate consumption and elongation growth, the so-called quiescent strategy (Fukao et al., 2006; Akman et al., 2012). Indeed, studies show that species with an escape strategy are prevalent on natural sites with frequent shallow and long-term flooding events, whereas those with a quiescent strategy are restricted to sites with deep or short-lasting floods (Voesenek et al., 2004).
Employment of an effective flooding survival strategy, either quiescence or escape, is thus crucial for plant competitive vigor and survival. Physiological studies have revealed that the gaseous plant hormone ethylene, which rapidly accumulates within flooded organs attributable to the reduced gas exchange underwater, is one of the main drivers regulating both strategies. Indeed, ethylene is considered to be the most reliable and earliest indicator of the flooded status of a plant (Voesenek and Sasidharan, 2013) because, as also shown here, internal oxygen levels can be quite high in submerged photosynthetic tissues, especially when sufficient illumination is present (Mommer et al., 2007). Ethylene can either stimulate or suppress growth, depending on the species (Voesenek et al., 1993a; Pierik et al., 2006; Nagai et al., 2010), and was also shown to be relevant in submergence survival strategies of rice (Oryza sativa; Xu et al., 2006; Hattori et al., 2009). Processes that mediate plant adaptation to flood-prone environments in wild species could represent a valuable source of tolerance mechanisms that are absent in current model species, but could be used to improve flooding tolerance in crops.
To this end, we investigated two wild species in which the quiescence and escape strategies have been extensively studied, Rumex acetosa (sorrel) and Rumex palustris (marsh dock), respectively. R. acetosa is rarely flooded in nature and these floods are generally short-lasting compared with R. palustris, which is frequently exposed to prolonged but shallow floods (Voesenek et al., 2004). Upon submergence, R. palustris orientates its leaves from a horizontal to vertical position (hyponastic growth), in an ethylene-dependent manner, and subsequently enhances the elongation rate of mainly the youngest petioles (Cox et al., 2006). In R. acetosa, this petiole elongation is suppressed by ethylene that accumulates during submergence (Voesenek et al., 1993a; Pierik et al., 2009). To survive floods, R. acetosa has to deal with limited gas exchange and must adjust its metabolism accordingly to limit energy and carbon use. To investigate the molecular processes underlying the adaptive growth differences between the two species, we used RNA sequencing (RNA-Seq) technologies to quantitatively analyze genome-wide transcript changes upon submergence. For each species, the transcriptome was analyzed in the youngest, most responsive petiole tissue when elongation differences attributable to submergence were pronounced.
Our data set required that we first perform a gene orthology analysis of wild Polygonaceae species in relation to other angiosperms. With this framework, we were able to identify genes that regulate the contrasting survival strategies of R. acetosa and R. palustris. We present evidence of effective metabolic adaptations in R. acetosa consistent with quiescence. For R. palustris, we demonstrate that submergence escape is associated with ethylene-mediated utilization of classic light responsive signaling pathways. We also show that petioles of this species, despite normoxic oxygen content in under illumination, upregulated hypoxia marker genes an ethylene-dependent manner. Thus, ethylene primes R. palustris to future low oxygen conditions.
RESULTS AND DISCUSSION
R. acetosa and R. palustris Have Contrasting Underwater Growth Responses Reflecting Their Natural Field Distribution
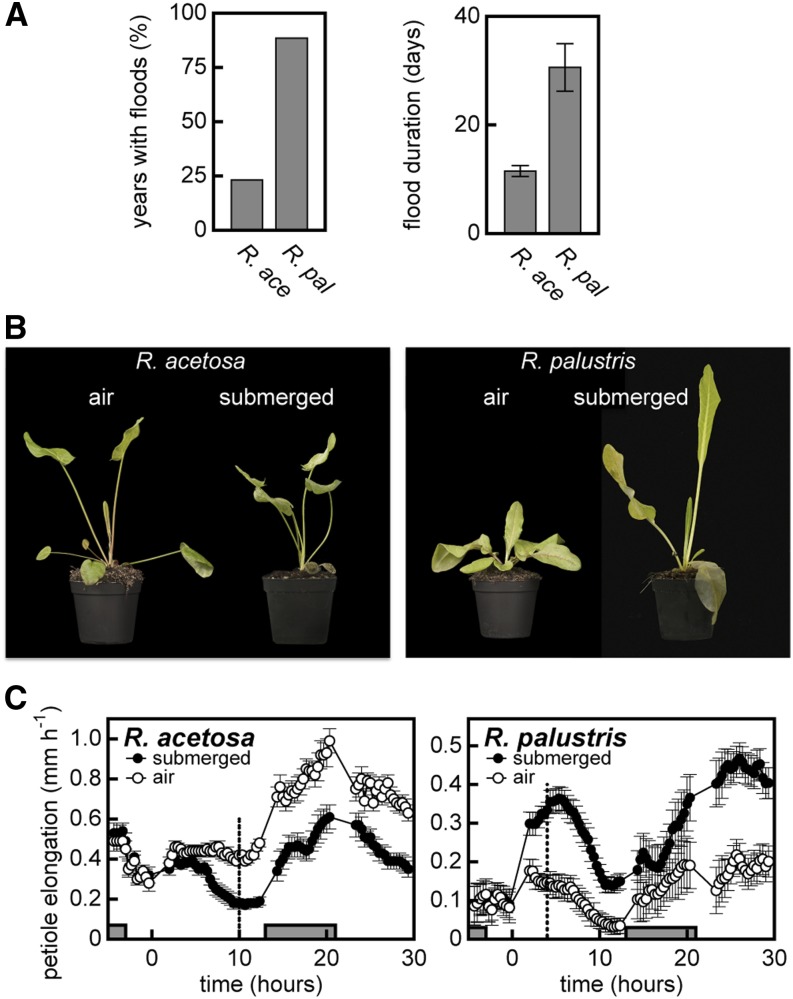
Both R. acetosa and R. palustris occur in the floodplains of major European rivers. The former inhabits the higher located grasslands, whereas R. palustris completes its life cycle in the lower located riparian mudflats (Van Eck et al., 2004). Accordingly, both the number of flooding events and their duration are higher in habitats of R. palustris than of R. acetosa (Figure 1A). Indeed, extended flooding periods have been shown to favor escaping species (Voesenek et al., 2004). In addition, the long duration and high frequency of flooding stress experienced by R. palustris has led to a strong selection for increased low oxygen and flooding tolerance of R. palustris compared with R. acetosa (Voesenek et al., 1993b). Nevertheless, both Rumex species have been found to be well adapted to flood-prone environments, with various reports of survival of more than 3 weeks for R. acetosa and even longer for R. palustris (Vervuren et al., 2003; Van Eck et al., 2004; Mommer et al., 2006a). A visual example of the contrasting survival strategies adopted in the Rumex species is shown in Figure 1B. Detailed analysis of the growth kinetics of the youngest petiole identified important lag phases and circadian patterns in the growth suppression of R. acetosa and stimulation of R. palustris (Figure 1C).
Figure 1.
Growth Strategies and Habitat of Two Wild Rumex Species.
(A) Flooding regime based on data from the Dutch Ministry of Infrastructure and the Environment over the period from 1970 to 1995 at elevation levels where R. acetosa (R. ace) or R. palustris (R. pal) are located. The left bar graph shows the percentage of years with flood occurrence in the growth season (April to September). The right bar graph presents the average (± sem) duration of the most extreme flooding event during the growth season of each year.
(B) Visual representation of the responses of R. acetosa (left) and R. palustris (right) after 10 d of complete submergence.
(C) Petiole elongation rate of the youngest developing leaf (third leaf) (mean ± sem; n = 11 for R. acetosa, n = 18 for R. palustris). Gray bars represent the dark period and the vertical dashed line indicates the sampling time-point for Illumina sequencing.
[See online article for color version of this figure.]
RNA-Seq Reveals Distinct Flooding Response Transcriptomes in Rumex
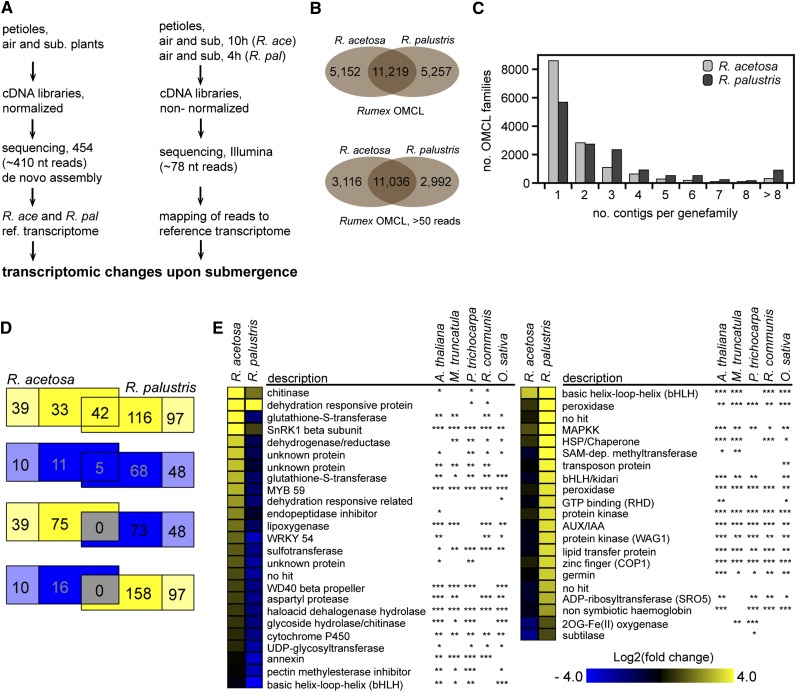
A two-step approach was taken to profile global transcriptome changes upon submergence in the two species. First, a de novo transcriptome was created using long-read sequencing (419 nucleotides), followed by identifying quantitative changes with short-read sequencing (78 nucleotides) at the time point slightly prior to maximum growth differences (Figure 2A). Using the youngest, most responsive petiole tissue from control or submergence-treated plants, the long-read sequencing resulted in de novo assembly of 32,022 R. acetosa and 49,070 R. palustris contigs (see Supplemental Table 1 online). The relatively low percentage of singletons (13%) indicated a well-assembled transcriptome (Ekblom and Galindo, 2011). Comparative gene family identification, using the Ortho Markov Clustering (OMCL) clustering algorithm (Enright et al., 2002; Li et al., 2003), among five angiosperms representing a wide range of phylogenetic lineages (Arabidopsis thaliana, Medicago truncatula, Solanum lycopersicum, Beta vulgaris, and O. sativa) revealed 4171 gene clusters unique to Rumex. Only 97 clusters were found in all species but the two Rumex species, whereas 4262 did include Rumex, indicating good sequence coverage and assembly using petiole mRNA (see Supplemental Figure 1 online). Identification of putative orthologs and putative paralogs specifically between R. acetosa and R. palustris yielded 16,476 and 16,371 clusters (OMCL families), respectively; of these, 11,219 contained contigs that were present in both species (Figure 2B). OMCL families were small and mostly contained only one or two contigs per species (Figure 2C). The small OMCL families allowed us to circumvent the lack of genome information while maintaining a high resolution of sequence diversity, limiting the potential pooling of contigs with different physiological functions. Interestingly, more contigs were identified in R. palustris and the number of OMCL families with more members was higher, which could reflect its polyploid nature compared with the diploid nature of R. acetosa (Darlington and Wylie, 1955) or differences in genetic variation of the experimental populations used.
Figure 2.
RNA-Sequencing Pipeline and Analysis.
(A) Experimental, analytical, and bioinformatics workflow used to characterize and quantify transcriptomic changes upon submergence.
(B) Distribution of OMCL families from R. acetosa and R. palustris of either all identified OMCL families, or those with more than 50 sequence reads in at least one treatment. The latter was used for subsequent analysis.
(C) OMCL family size distribution of families with more than 50 sequence reads in at least one treatment.
(D) Venn diagram of differentially expressed OMCL families upon submergence, where yellow is up, blue is down, and gray the overlap between upregulation and downregulation (adjusted P < 0.01). The pale colors are OMCL families specific to either R. acetosa or R. palustris.
(E) OMCL families with a strong species × treatment interaction (adjusted P < 0.01). Annotation of the OMCL families was based on a discontiguous megablast against five other plant species. *Eval > 10−10; **10−10 < Eval < 10−30; ***Eval < 10−30.
To identify molecular processes that mediate submergence-induced petiole growth stimulation and suppression, sampling for quantitative short-read RNA-Seq was based on time points just prior to the strongest effect of submergence on petiole elongation. This was after 10 h for R. acetosa and 4 h for R. palustris. Short-read RNA-Seq of air- or submergence-treated petiole tissue from these time points delivered ∼30 million reads per sample. Of these, 74% and 77% of the reads could be mapped to the reference transcriptome of R. acetosa and R. palustris, respectively (see Supplemental Table 1 online). By summing the reads of all OMCL family contig members, differential expression analysis based on a negative binomial model identified 469 differentially expressed OMCL families (adjusted P < 0.01) (Figure 2D; see Supplemental Figure 1C online). Quantitative RT-PCR on the same samples yielded results consistent with RNA-Seq (see Supplemental Figure 1D online). Large-scale changes upon submergence were also characterized by analyzing overrepresentation of Gene Ontology (GO) terms among OMCL families with estimated log2FC (for fold change) exceeding |1| (see Supplemental Figure 2 online). Distinct and overlapping transcriptomic responses of both individual OMCL families and GO categories between the two species were found (Figure 2D; see Supplemental Figure 2 online). However, no case of opposing regulation (i.e., up in one species while down in the other) was identified; rather, different cohorts of differentially expressed OMCL families and GO categories are regulated in either species (Figure 2D; and see Supplemental Figure 2 online). This suggests that at least the transcriptional regulation of growth stimulation and suppression are controlled by distinct sets of genes instead of being contrastingly regulated by a few master regulators. A full factorial generalized model identified 46 OMCL families with a significant species × treatment interaction term (adjusted P < 0.01; Figure 2E). These are candidate regulators of the distinct plasticity in growth of the two species during submergence.
Transcriptomic Overlap between R. acetosa and R. palustris identifies Processes Related to Carbon Starvation, Toxins, and Ion Homeostasis Typical of Underwater Stress
The overlap between R. acetosa and R. palustris in regulated OMCL families and overrepresented GO terms are likely to be common responses of Rumex to the physiological challenges of the underwater environment. For example, this is demonstrated by the induction of putative orthologs of DARK INDUCIBLE10 (fam02035, fam06971), which typically occurs upon carbon starvation in Arabidopsis, where they encode hydrolases that act on sugar polymers (Fujiki et al., 2000, 2001). In addition, the upregulation of β-amylase transcripts was observed (fam18839, fam08330). Overall changes identified by a GO analysis further supported general metabolic changes in both species as reflected by the overrepresentation of carbohydrate metabolic process (GO:0005975), hydrolase activity (GO:0004553), and triglyceride catabolic process (GO:0019433). In addition, an overrepresentation of GO terms associated with energy-requiring processes such as DNA replication (GO:0006270, GO:0006268) and flavonoid biosynthesis (GO:0009813) among the downregulated genes was identified. The physical challenge of reduced gas exchange underwater is reflected in these metabolic adjustments in which both species appear to downregulate energy-consuming processes and activate reserve mobilization. The impairment of photosynthesis underwater generally leads to metabolic imbalances and thus toxic end products and reactive oxygen species (ROS) formation. Concomitantly, commonly regulated OMCL families included two lactoylglutathione lyases (fam15402, fam16911) and a glutathione-S-transferase (fam12707), which all function in detoxification processes (Moons, 2005). Furthermore, a drug transporter (fam20272) was strongly induced and there was a strong overrepresentation of the toxin catabolic GO term (GO:0009407; see Supplemental Figure 2 online). Both R. acetosa and R. palustris induced some ROS-associated transcripts, such as a putative ortholog of the Arabidopsis OXIDATIVE STRESS3 (fam21461) and a peroxidase (fam15244). However, R. palustris activated an additional set of ROS-associated genes such as two more peroxidases (fam10911, fam19976) and glutathione biosynthetic enzymes (fam04087), suggesting enhanced ROS regulation. This might be an anticipatory response for the moment when reaeration occurs when the water surface is reached via the elongation response. Alternatively, some photosynthetic capacity of submerged R. palustris has been reported (Mommer et al., 2006b), whereas R. acetosa does not benefit as much from underwater illumination (Mommer et al., 2006a). Therefore, the additional activation of ROS and redox players might be important to appropriately adjust redox regulation.
The GO analysis also indicated strong regulation of transcripts important for water and ion homeostasis. This was also reflected in the shared activation of a Pro dehydrogenase associated with osmotic stress (fam06756), and many transmembrane and ion transport terms were overrepresented in both species (GO:0015297, GO:0015238, GO:0005451, GO:0015385, GO:0030104, GO:0006885, GO:0055085; see Supplemental Figure 2 online). Under normal drained conditions, plants are supplied with water and nutrients via the root system; during submergence, water and nutrient uptake is severely reduced as a result of an impaired transpiration stream and severely impaired root functioning (Elzenga and van Veen, 2010). However, aquatic and submerged plants do retain some capacity for water transport (Pedersen, 1993). The observed changes in ion homeostasis in Rumex most likely reflect the need to adjust to changes in nutrient supply. Changes in ion regulation are thus far an underestimated aspect of flooding tolerance (Shabala, 2011). Together with detoxification and metabolic adjustments, these are typical responses that deal with physical limitations of the underwater environment encountered by both species.
Hormonal Regulation of Petiole Growth in R. acetosa and R. palustris
Previous work on the regulation of hyponastic growth and petiole elongation of R. palustris compared with the quiescence response of R. acetosa revealed a crucial role of an ethylene-driven signaling cascade. In R. palustris, ethylene accumulation reduced abscisic acid (ABA) levels, auxin redistribution within the plant, and cell wall loosening (Cox et al., 2004; Benschop et al., 2005; Vreeburg et al., 2005). Continuation of elongation was shown to then require subsequent enhancement of gibberellic acid (GA) biosynthesis (Benschop et al., 2006). By contrast, submerged R. acetosa maintains ABA and GA at the same levels as control plants (Benschop et al., 2005). In addition, upon submergence, GA sensitivity is reduced in R. acetosa but enhanced in R. palustris, accentuating the contrasting behavior of these species (Rijnders et al., 1997). Accordingly, we investigated the underlying transcriptomic responses in these hormonal changes (see Supplemental Figure 3 online).
As expected, this transcriptome-wide analysis showed that aminocyclopropanecarboxylate oxidase, a proxy for enhanced ethylene levels (Vriezen et al., 1999), accumulated in both species. A strong induction of an EIN3 BINDING F-BOX (EBF) OMCL family was observed in R. palustris only. The putative ortholog in rice is likely involved in ethylene-induced growth stimulation by preventing negative regulation of GA synthesis by ethylene (Kim et al., 2012). This function is in line with the observed increase in EBF expression in the elongating R. palustris.
Consistent with previous observations (Benschop et al., 2005), transcripts encoding the rate-limiting ABA biosynthesis enzyme 9-cis-epoxycarotenoid dioxygenase were strongly downregulated in R. palustris and not in R. acetosa. In line with the decrease of ABA levels in R. palustris (Benschop et al., 2005), the gene transcript that encodes an ABA breakdown enzyme ABA-8-hydroxylase (a cytochrome P450 monooxygenase) was induced. Surprisingly, this was also observed in R. acetosa, although a different OMCL family of ABA-8-hydroxylases showed this regulation (see Supplemental Figure 3 online). Furthermore, a strong induction of the ABA receptor OMCL family PYR/PYL/RCAR (PYRABACTIN RESISTANCE/PYR1-LIKE/REGULATORY COMPONENT OF ABA RECEPTOR) was found in both species, whereas OMCL families encoding orthologs of two downstream components of ABA signaling (ABA responsive element binding factor2 and HOMEOBOX PROTEIN33) were induced only in R. acetosa (see Supplemental Figure 3 online) (Kim et al., 2004; Wang et al., 2011). This indicates enhanced ABA signaling, possibly via the combination of maintained ABA levels and elevated receptor levels that could facilitate growth suppression in R. acetosa. No strong changes were observed in either species in transcripts encoding GA biosynthetic enzymes, GA receptors, or DELLA proteins (see Supplemental Figure 3 online). This is in line with previous physiological studies showing that GA is not an early signal in enhanced petiole elongation, because significant increases in GA1 content in R. palustris were observed only after 6 h of submergence (Benschop et al., 2006). However, an overrepresentation was found for GA-mediated signaling in R. palustris (see Supplemental Figure 2 online), suggesting a sensitization to GA prior to the increase in actual GA levels. This early sensitization could potentially act via the observed changes in ABA signaling transcripts as an important antagonist to GA signaling in Rumex (Benschop et al., 2006; Chen et al., 2010).
A large effect was also seen on auxin transport–modulating OMCL families. These included transcripts encoding putative orthologs of a PINOID like (WAG1) (Santner and Watson, 2006), PINOID BINDING PROTEIN1 (Benjamins et al., 2003) and quercetin-O-glucosyltransferases (Jacobs and Rubery, 1988). Furthermore, transcripts of AUXIN (INDOLE-3-ACETIC ACID)2-11 putative orthologs, a typical auxin responsive gene (Wyatt et al., 1993), were regulated only in R. palustris. These transcriptome changes are consistent with the previously identified role of auxin transport and abundance in the early hours of submergence-induced petiole elongation (Cox et al., 2006).
Putative downstream genes of these hormonal interactions, such as the cell wall–modifying expansins, are induced in R. palustris and not in R. acetosa (Vreeburg et al., 2005). These include the previously identified expansin RpEXPA1 (fam00649) as well as xyloglucan endotransglucosylase-hydrolases, and pectinesterases, supporting the conclusion that submergence induces the full suite of cell wall–modifying players (Sasidharan et al., 2011) in R. palustris, but not R. acetosa.
Rumex Species Reconfigure Primary Metabolic Pathways in Response to Submergence
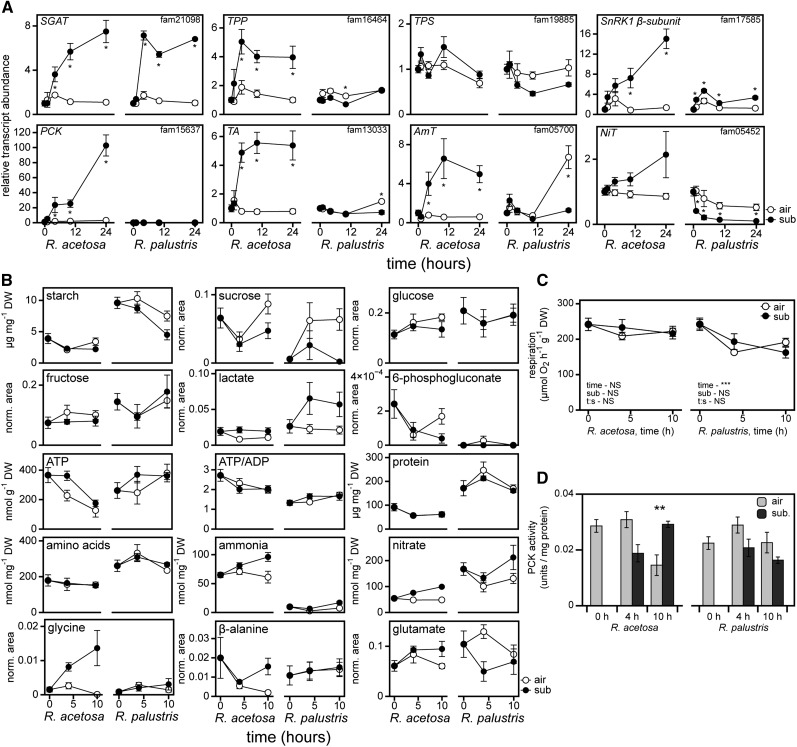
The RNA-Seq dataset suggested that distinct changes in carbon and nitrogen metabolism occur especially in R. acetosa. Among the OMCL families with strong species × treatment effects, R. acetosa strongly induced transcripts encoding the β-subunit of the heterotrimeric SNF1-related kinase 1 (SnRK1) complex, together with a range of OMCL families encoding metabolic enzymes, whereas R. palustris showed considerably less induction of the same OMCL families (Figure 2E; see Supplemental Figure 4 online). Further investigation into OMCL families involved in metabolism supported the notion of specific metabolic responses in R. acetosa (see Supplemental Figure 4 online). We therefore performed directed analyses of submergence-induced changes in transcripts encoding relevant metabolic enzymes, metabolites, and oxygen consumption in both species (Figure 3, Table 1; see Supplemental Figure 5 online).
Figure 3.
Changes in Metabolism upon Submergence.
(A) Relative gene expression profiles in petioles from submerged plants (mean ± sem, n = 6; *P < 0.05, Tukey least significant difference [LSD]). SGAT, Ser:glyoxylate aminotransferase2; TA, Thr aldolase; AmT, ammonium transporter; NiT, nitrate transporter.
(B) Changes in levels of selected metabolites upon submergence in Rumex petioles (mean ± sem, n = 5). Data for other measured metabolites are provided in Supplemental Figure 5 online. Significance levels for all metabolites are presented in Table 1.
(C) Respiratory rates, measured on excised petioles via consumption of oxygen in the dark (mean ± sem, n = 4). ***P < 0.001, two-way analysis of variance. NS, no significant effect; sub, submergence main effect; time, time main effect; t:s, time × submergence interaction
(D) PCK activity of petioles from air or submerged plants (mean ± sem, n = 5).
Table 1. Identified Metabolites and Their Behavior during Submergence.
| Metabolite |
R. acetosa |
R. palustris |
||||||
|---|---|---|---|---|---|---|---|---|
| Submergence (P value) | Time (P value) | t × s (P value) | Direction | Submergence (P value) | Time (P value) | t × s (P Value) | Direction | |
| Suc | <0.01 | <0.01 | Down | |||||
| Glc | ||||||||
| Fru | ||||||||
| 6-phosphogluconate | <0.05 | Down | ||||||
| 3-phosphoglycerate | <0.01 | |||||||
| Citrate | ||||||||
| α-ketoglutarate | ||||||||
| Succinate | <0.01 | |||||||
| Malate | ||||||||
| Oxalate | <0.01 | |||||||
| Glu | <0.05 | Down | ||||||
| Pyroglutamate | ||||||||
| Aspartate | <0.01 | |||||||
| Gly | <0.05 | <0.05 | Up | |||||
| β-Ala | <0.01 | Up | ||||||
| Lactate | <0.01 | Up | <0.05 | Up | ||||
| Myo-inositol | ||||||||
| Ascorbate | ||||||||
| β-sitosterol | <0.05 | Up | ||||||
| Campesterol | <0.05 | Up | ||||||
| Shikimate | <0.05 | |||||||
| Tartrate | <0.05 | |||||||
| Propanoate | <0.01 | |||||||
| Erythronate | <0.01 | <0.05 | Up | |||||
| Total protein | <0.001 | |||||||
| Amino acids | <0.05 | |||||||
| Nitrate | <0.001 | <0.01 | <0.001 | Up | ||||
| Ammonia | <0.01 | Up | <0.001 | <0.001 | <0.05 | Up | ||
| Starch | <0.05 | Down | <0.05 | <0.001 | Down | |||
| ATP | <0.05 | <0.001 | Up | |||||
| ATP:ADP | ||||||||
Data presented as P values were calculated using two-way analysis of variance. Blank cells indicate no significant effect. Submergence, submergence main effect; Time, time main effect; t × s, time × submergence interaction. Direction, direction of change, either down or up indicating lower or higher levels in submerged plants.
Gas exchange is restricted in underwater leaves and this compromises photosynthesis (Mommer and Visser, 2005). Under such conditions, reduced availability of CO2 would also promote photorespiration, as indicated by transcriptional activation of Ser:glyoxylate amino transferase in both species (Figure 3A; see Supplemental Figure 4 online). This is likely to induce carbon limitation. However, energy levels might not be compromised because shoots often remain normoxic when submergence occurs in the light (Mommer et al., 2007). Accordingly, ATP levels and respiration were not negatively affected by submergence compared with controls in both species (Figures 3B and 3C). We also found that both species had increased lactate levels (Figure 3B), suggesting that fermentation occurs in submerged petioles or alternatively that lactate produced in hypoxic roots is transported to aerial tissue. Despite these observed similarities, metabolic requirements are expected to be distinct between the two species. Although R. palustris requires resources to sustain elongation growth and regain aerial contact, growth suppression upon flooding would require R. acetosa to adapt its metabolism to a situation in which no aerial contact will be made. Although the two species were distinct in their metabolite levels prior to submergence, we discerned species-specific trends in metabolite and transcript changes in response to submergence (Figure 3, Table 1; see Supplemental Figure 4 online).
Although many reserve-mobilizing components were induced in both species, such as β-amylases and two sugar hydrolytic DARK INDUCIBLE OMCL families, R. acetosa activated an additional suite of catabolic reactions absent in R. palustris, such as gene transcripts associated with starch, Lys, and Thr catabolism (see Supplemental Figures 2 and 4 online). A trehalose phosphate phosphatase (TPP) was induced throughout submergence only in R. acetosa (Figure 3A). Its substrate trehalose-6-phosphate (T6P) is a potent growth regulator that acts by inhibiting the activity of the SnRK1 heterotrimeric protein complex (Zhang et al., 2009). Assuming that TPP transcriptional elevation translates to higher TPP activity, the consequent decrease in T6P and subsequent release of the inhibition on SnRK1 activity would lead to a shift from energy-consuming anabolism toward energy-producing catabolism (Baena-González et al., 2007). Furthermore, transcriptional induction specific to R. acetosa of Suc phosphate synthase and phosphoenolpyruvate carboxykinase (PCK), the latter one of many targets activated by SnRK1 (Baena-González and Sheen, 2008), indicates additional species-specific metabolic changes. Overall, the activation of a catabolic suite of genes in combination with reduced biosynthesis would lead to the maintenance of major metabolite pools (Suc, hexoses, starch, tricarboxylic acid [TCA] cycle intermediates, proteins, and amino acids) (Figure 3B, Table 1). A similar observation was made in rice, in which a quiescent variety showed increased TPP transcript accumulation and relatively high sugar status compared with a nonquiescent genotype (Jung et al., 2010; Barding et al., 2012). Overall, this suggests an uncoupling of carbon status and trehalose metabolism in quiescent species, which would allow for maintenance of high free sugar levels without engaging in expensive biosynthetic processes. Accordingly, submerged R. acetosa had significantly lower levels of 6-phosphogluconate (Figure 3B), an intermediate in the oxidative pentose phosphate pathway that supplies potent reducing power for biosynthesis. By contrast, R. palustris, which requires carbon building blocks for elongation growth, suffered from a depleted Suc pool and reduced starch levels (Figure 3B).
The β-subunit of the SnRK1 complex, with a strong transcriptional induction in R. acetosa, has been shown to have a specific role in regulating nitrogen metabolism (Li et al., 2009). Combined with the simultaneous induction of TPP, which presumably relieves T6P inhibition on SnRK1, would suggest SnRK1-mediated nitrogen metabolic reprogramming upon submergence in R. acetosa. Indeed, several GO terms associated with amino acid metabolism were overrepresented in R. acetosa only, such as Arg and Lys transmembrane transport, Lys catabolic process, regulation of nitrogen compound, and ammonium transporters (GO:0015181, GO:0051589, GO:0019477, GO:0051171, GO:0015696; see Supplemental Figures 2 and 4 online). Despite the catabolic nature of the transcriptional changes, the overall amino acid and protein levels did not change in either species. However, certain amino acid levels changed in a species-specific manner. Submergence increased Gly and Ala levels in R. acetosa and reduced Glu levels in R. palustris (Figure 3B). In R. acetosa, the strong Gly accumulation corresponded with an increase in threonine aldolase expression, which converts Thr to Gly and acetaldehyde (Figure 3B). Acetaldehyde could serve as a substrate for fermentation or be metabolized to acetyl-CoA. Interestingly, a significant increase of free ammonia was observed in submerged R. acetosa (Figure 3B). Thus far, this has not been observed in submerged plants and a clear functional explanation remains absent. However, this could be the result of reduced biosynthesis or increased breakdown of protein and amino acids in this species, especially given that a minor change in protein content could already result in a dramatic increase in ammonia or nitrate.
Interestingly, two PCKs were among the most strongly induced OMCL families in R. acetosa (fam06080, fam15637; log2FC = 7.09 and 6.55). Indeed, real-time quantitative PCR analyses revealed significant elevation of PCK transcripts over time in submergence-treated R. acetosa samples (Figure 3A). Accordingly, in vitro PCK activity was higher in submerged R. acetosa (Figure 3D). PCK was shown to be strongly induced by feeding certain amino acids, ammonia or nitrate (Delgado-Alvarado et al., 2007), and PCK knockout plants were shown to have altered amino acid profiles (Brown et al., 2010). PCKs take a central part in metabolism by converting oxaloacetate from the TCA cycle to phosphoenolpyruvate. This reaction is especially important in gluconeogenesis and the subsequently formed phosphoenolpyruvate acts as a potent inhibitor of phosphofructokinase activity, thus limiting glycolysis. However, we observed no reduction in levels of downstream components such as TCA cycle intermediates (Table 1) or respiration (Figure 3C). PCK activation and ammonia transporters (Figure 3B) have been suggested to be required for removal of acids and ammonia after amino acid breakdown (Leegood and Walker, 2003).
In summary, our observations support distinct metabolic changes in response to submergence for R. acetosa compared with R. palustris. Directing resources toward appropriate processes and initiating the right metabolic adjustments is critical for survival of any stress. In R. palustris, metabolic adaptation is restricted to increased reserve mobilization, probably to fuel elongation growth. In R. acetosa, submergence-induced transcriptional and metabolic signatures that suggest changes in carbon and nitrogen metabolism that mediate management of energy resources such as via the arrest of biosynthetic processes.
Shade Avoidance and Photomorphogenesis Are Regulated by Ethylene, Not Light, during Submergence
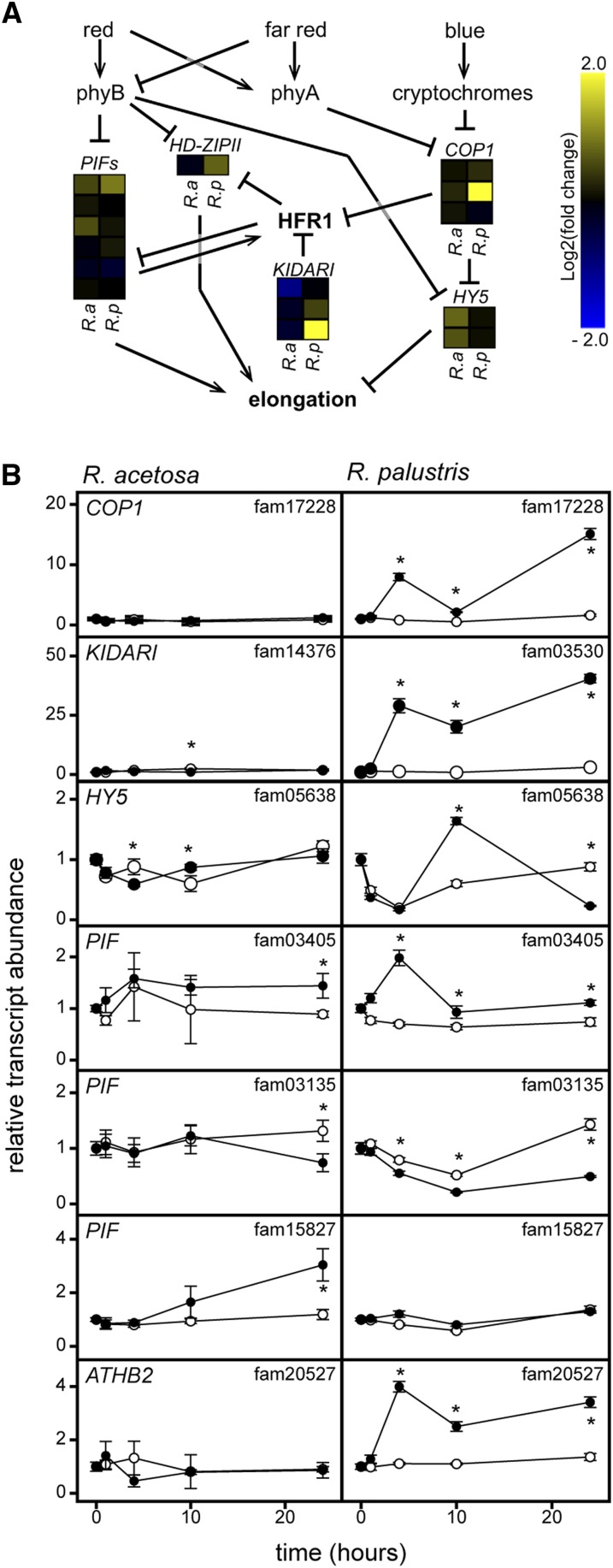
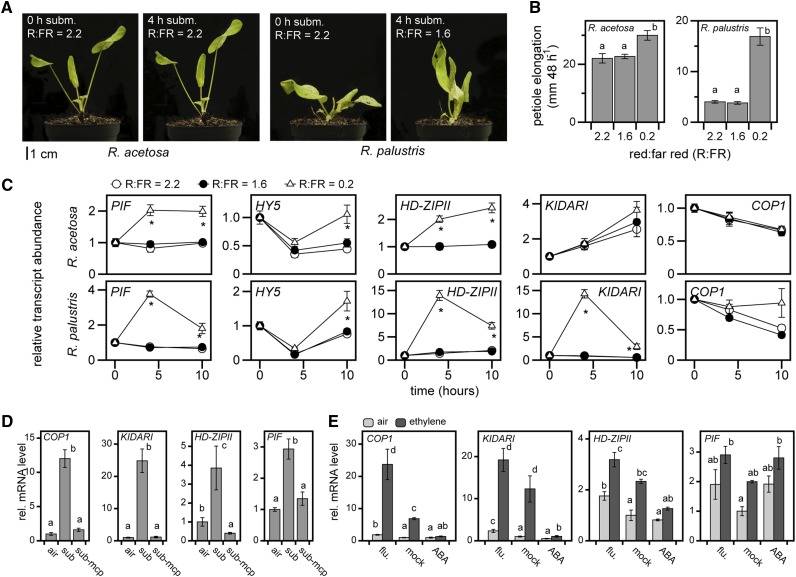
Further investigation of the OMCL families with a strong interaction effect between species and treatment revealed several genes associated with photomorphogenesis and shade avoidance (Figure 2E). This was also reflected in the GO analysis by overrepresentation of the shade avoidance term only among upregulated R. palustris OMCL families (see Supplemental Figure 2 online). These include putative orthologs of Arabidopsis AUXIN /INDOLE-3-ACETIC ACID INDUCIBLE 2-11(AT5G43700) (Wyatt et al., 1993), zinc finger CONSTITUVE PHOTOMORPHOGENIC1 (COP1), and the basic helix-loop-helix (bHLH) transcription factor gene KIDARI/PRE6. Both COP1 and KIDARI are involved in light regulation of elongation growth via interaction with LONG HYPOCOTYL IN FAR-RED1 (HFR1), an atypical bHLH that regulates shade avoidance. HFR1 can form heterodimers with phytochrome interacting factors (PIFs), thereby preventing their binding to promoters of target genes that are associated with enhanced shoot elongation (Hornitschek et al., 2009; Galstyan et al., 2011). COP1 can function as an E3 ligase and targets a range of proteins involved in photoreceptor-mediated signal transduction for degradation, including HFR1 (Duek et al., 2004; Jang et al., 2005; Yang et al., 2005). KIDARI also negatively regulates HFR1 action, but does so by forming heterodimers and thereby releasing PIFs from HFR1 inhibition (Hyun and Lee, 2006; Lee et al., 2006). The PIFs directly interact with phytochrome photoreceptors and thus act downstream of red and far-red stimuli (Leivar et al., 2012). PIF4 and PIF5 in Arabidopsis also stimulate their direct negative regulator HFR1, thereby ensuring a fine-tuned elongation response during shading (Lorrain et al., 2008; Hornitschek et al., 2009). In addition to COP1 and KIDARI, several other shade avoidance–associated genes were upregulated in submerged R. palustris, including PIFs, the negative regulator of elongation growth ELONGATED HYPOCOTYL5 (HY5) (Osterlund et al., 2000; Li et al., 2010), and a class II homeodomain-leucine zipper (HD-ZIPII), a family of positive regulators of shade avoidance (Steindler et al., 1999) (Figure 4A). Quantitative RT-PCR verified the induction and changes of KIDARI, COP1, PIF, and HD-ZIPII over a 24-h period in R. palustris only (Figure 4B). Based on knowledge from Arabidopsis, the combined and strong regulation of KIDARI and COP1 transcripts would indirectly act via a release of PIF suppression and the transcriptional activation of PIF and HD-ZIPII. These results suggest that these OMCL families, typical of photomorphogenesis and shade avoidance, are also employed in submergence-induced elongation of petioles of R. palustris (Figure 4).
Figure 4.
Light Signaling Components Regulated during Submergence in Rumex
(A) Model of interactions between molecular components and photoreceptors that regulate elongation growth based on studies in Arabidopsis. Family identification numbers are as follows: HD-ZIPII, 20527; PIFs, 03405, 15415, 15827, 01516, 03135, 08791; KIDARI, 14376, 10925, 03530; COP1, 15699, 17228, 06784; HY5, 09886, 05638.
(B) Time series expression profile of seven OMCL families under air or submerged conditions for R. acetosa and R. palustris (mean ± sem, n = 5; * P < 0.05, Tukey LSD).
As mentioned, R. palustris reorientates its leaves vertically (hyponasty) upon submergence. This can result in horizontal far-red reflection from one leaf to another, much the same way as in dense canopies where this is an early signal for shade avoidance (de Wit et al., 2012). Measurements of the changes in the red to far-red ratio (R:FR) at the third leaf, which has the strongest elongation response, revealed that there was indeed a reduction in the R:FR from 2.2 (standard growth chamber light quality) to 1.6, indicating such far-red reflection, in R. palustris and not in R. acetosa (Figure 5A). However, experimentally reducing R:FR to 1.6 by supplying additional far-red light was not sufficient to induce an elongation response or to induce the expression of a set of photomorphogenesis genes under nonsubmerged conditions in either species. Nonetheless, both R. palustris and R. acetosa were able to respond to a strong reduction in R:FR from 2.2 to 0.2 via petiole elongation and marker gene expression (Figures 5B and 5C). Furthermore, artificial filtering of far-red wavelengths, resulting in high R:FR ratios underwater, did not alter the elongation response in R. palustris (see Supplemental Figure 6 online).
Figure 5.
The Role of Light Quality and Hormonal Changes in Underwater Growth.
(A) Photographs demonstrating the typical hyponastic response of R. palustris upon submergence and the lack of this response in R. acetosa. R:FR is measured between hyponastic lamina or at the lamina surface under submerged or air conditions.
(B) Petiole elongation under various R:FR treatments (ambient, 2.2; submergence mimic, 1.6; and very low R:FR, 0.2) of the youngest leaf (third leaf) over a 48-h period (mean ± sem, n = 12; P < 0.05, Tukey LSD). Different letters above each bar indicate statistically significant differences.
(C) Rumex OMCL family transcript accumulation of key regulators of light modified elongation in petioles upon three R:FR levels (mean ± sem, n = 5; *P < 0.05, Tukey LSD). PIF is fam03405.
(D) Effect of the ethylene action inhibitor 1-methylcyclopropene (1-MCP) during submergence on the expression of four light signaling genes in R. palustris after 4 h of treatment (mean ± sem, n = 5; P < 0.05, Tukey LSD). Different letters above each bar indicate statistically significant differences.
(E) The effect of ethylene and ABA manipulations on the expression of four light-signaling genes in R. palustris after 4 h of treatment (mean ± sem, n = 4; letters P < 0.05, Tukey LSD). Flu is the ABA biosynthesis inhibitor fluridone.
[See online article for color version of this figure.]
Our data suggest that the elongation response of R. palustris employs transcripts associated with photomorphogenesis and shade avoidance. Because exposing nonsubmerged plants to the R:FR conditions that occur in hyponastic, submerged plants failed to alter gene expression as well as elongation growth (Figure 5B and 5C), we conclude that R. palustris activates the light signaling machinery underwater independently of phytochromes. Indeed, the inhibition of ethylene perception by the ethylene receptor inhibitor 1-methylcyclopropene demonstrated that ethylene action is required for the activation of COP1, KIDARI, HD-ZIPII, and PIF during submergence (Figure 5D). Furthermore, the downregulation of ABA by ethylene that normally occurs in R. palustris during submergence is probably essential for transcriptional induction of COP1 and KIDARI, because ABA addition blocked their ethylene-induced expression (Figure 5E). ABA content was not downregulated in R. acetosa upon submergence (Benschop et al., 2005), thus explaining the lack of regulation of photomorphogenesis genes in this species.
We suggest that in R. palustris, shade cues and submergence converge on a similar signal transduction pathway (Pierik et al., 2011). A possible scenario for control of submergence-induced shoot elongation in R. palustris would therefore be that ethylene accumulation, resulting in ABA downregulation (Benschop et al., 2005), stimulates the expression of KIDARI and COP1. The proteins encoded by these transcripts stimulate PIF action and therefore elongation growth. Simultaneously, HD-ZIPII also requires ethylene-mediated ABA degradation for its transcriptional activation, likely further enhancing elongation growth. Downstream of these modules several other regulators, such as GA, might be modulated. Indeed, GA levels in R. palustris start to increase after 6 h of submergence (Benschop et al., 2006). The ethylene-driven activation of photomorphogenesis and shade avoidance–associated genes appears not to occur during flooding-induced shoot elongation in lowland rice varieties, in which no shade avoidance or photomorphogenesis genes were identified among the differentially expressed genes from a transcriptome-wide analysis (Jung et al., 2010). With the strong induction of KIDARI and COP1 in R. palustris and not in R. acetosa, and with their light-independent regulation, we suggest that these genes are regulators of the underwater elongation response using a conserved signal transduction network that is also activated in response to shade.
Ethylene Primes R. palustris, but not R. acetosa, for Upcoming Anoxia
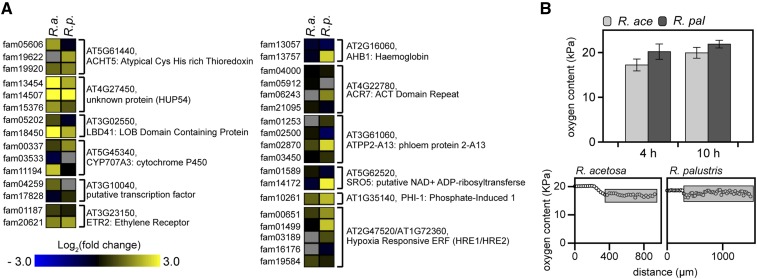
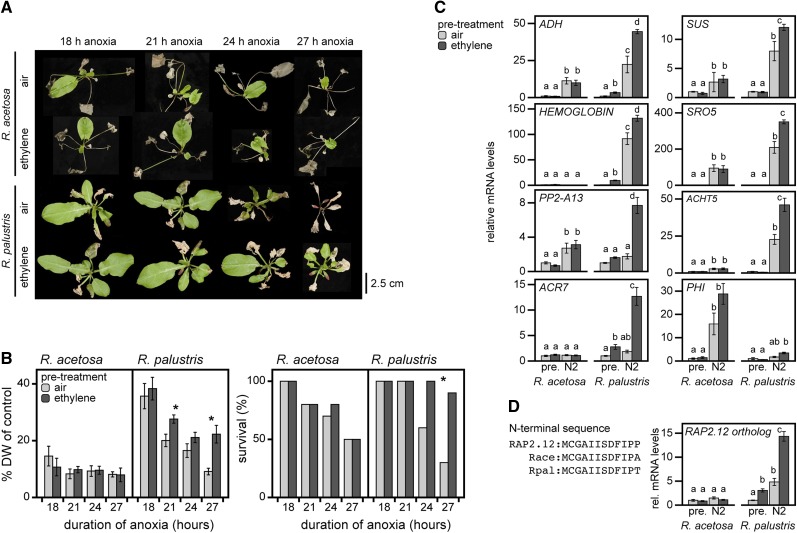
Among the OMCL families that showed the strongest contrasting behavior between R. acetosa and R. palustris (Figure 2E) were two that are typical markers for low oxygen: nonsymbiotic hemoglobin (HB) and a NAD+ ADP-ribosyltransferase (SRO5). These are part of a group of 49 core hypoxia-response genes widely upregulated in response to oxygen deprivation in the plant kingdom and in Arabidopsis seedlings independent of organ or cell type (Mustroph et al., 2009, 2010). A discontiguous megablast of the Arabidopsis core set revealed the Rumex equivalent OMCL families (Eval < 1.0E-10; see Supplemental Figure 7 online). OMCL families of 30 of the 49 core hypoxia genes in Arabidopsis were identified in Rumex, 4 of which were induced in both Rumex species by submergence and an additional 7 were upregulated in R. palustris only (Figure 6A). Interestingly, despite induction of numerous hypoxia core set OMCL families, oxygen levels inside petioles as measured by microelectrodes did not drop below 17% in either species during submergence in the light (Figure 6B). Oxygen content in submerged tissue is typically light dependent (Mommer et al., 2007). Microelectrode measurements determined that Rumex petioles indeed showed a severe depletion of oxygen levels when submergence occurred in the dark (see Supplemental Figure 7 online). On the basis of this finding, we hypothesized that the induction of hypoxia-responsive genes in the Rumex petioles during normoxia acts as a mechanism to prime the plant to a potential imminent oxygen crisis associated with flooding. This would occur if aerial contact was not restored by escape of leaves above the water surface, if floodwaters were turbid, or as a consequence of nightfall. Because ethylene entrapment is an inevitable consequence of submergence, we hypothesized that elevated ethylene might be responsible for the induction of these hypoxia-responsive genes. To test this, a 4-h ethylene pretreatment was applied followed by complete anoxia in darkness for 18 to 27 h. In this time window, both species were compromised in plant survival. Nevertheless, pretreatment of R. palustris with ethylene significantly enhanced tolerance to subsequent anoxia, whereas in the ethylene pretreatment had no significant effect R. acetosa (Figures 7A and 7B). The increased survival of R. palustris was accompanied by a smaller reduction of shoot dry mass compared with untreated plants.
Figure 6.
Regulation of Typical Hypoxia OMCL Families Despite High Oxygen Conditions.
(A) The homologs of the Arabidopsis core hypoxia gene set that were differentially regulated upon submergence. For most Arabidopsis genes, several homologous OMCL families were found (Eval < 1.0E-10). R.a. = Rumex acetosa; R.p. = Rumex palustris.
(B) Oxygen content in the Rumex petioles upon complete submergence under light conditions (mean ± sem, n = 3). Representative traces for both species are given, where the shaded areas mark measurements inside the petioles.
Figure 7.
Ethylene Primes R. palustris for Upcoming Anoxic Conditions.
(A) Rumex phenotypes after 4 h of normoxic pretreatment with or without ethylene, followed by anoxia (N2) treatment in the dark (18-27 h) and 7 d of recovery under standard growth conditions.
(B) Effect of pretreatment and anoxia on dry weight (DW) (mean ± sem, n = 10; * P < 0.05, Tukey LSD) and plant survival (n = 10, * P < 0.05, chi square). The experiment was repeated three times independently with similar results (representative data are shown).
(C) Expression of eight OMCL families that are identified as core hypoxia-responsive genes in Arabidopsis after either 4 h of pretreatment (air or ethylene) or pretreatment in combination with 4 h of anoxia (n = 5, mean ± sem; letters P < 0.05 Tukey LSD).
(D) The conserved N terminus essential for oxygen sensitivity of Arabidopsis RAP2.12 and the Rumex orthologs, together with the relative transcript abundance of the Rumex orthologs (letters P < 0.05, Tukey LSD) upon ethylene (pretreatment 4 h) and subsequent anoxia (N2, 4 h.).
[See online article for color version of this figure.]
Consistent with the observed survival differences between R. acetosa and R. palustris, none of the core hypoxia genes were induced by ethylene in R. acetosa, whereas ALCOHOL DEHYDROGENASE (ADH), HB, and ACT DOMAIN REPEAT7 transcripts were elevated by ethylene pretreatment in R. palustris (Figure 7C). Moreover, six of the eight hypoxia core set gene families tested showed greater induction during anoxia in R. palustris plants pretreated with ethylene compared with air pretreatment. In contrast, none of the investigated hypoxia core set OMCL families showed ethylene pretreatment enhanced expression in R. acetosa during anoxia. Remarkably, these gene transcripts showed little or no change in abundance as a result of ethylene pretreatment alone. Taken together, these data indicate that ethylene pretreatment primes the expression of several hypoxia core set members.
The recent characterization of group VII ethylene responsive transcription factors (ERFs) whose stability is dependent on oxygen availability (Gibbs et al., 2011; Licausi et al., 2011) provides a possible mechanism via which such priming effects could occur. Under normoxic conditions, the Arabidopsis group VII ERF RAP2.12 binds a plasma membrane bound protein thus preventing its oxygen-dependent degradation via the N-end rule of targeted proteolysis (Licausi et al., 2011). Upon transfer to anoxic conditions, RAP2.12 is released from the membrane, stabilized, localized to the nucleus, and directly or indirectly activates hypoxia-responsive genes such as ADH1. Indeed, there was a modest upregulation of a RAP2.12 OMCL family in submerged R. palustris (fam00651; log2FC = 1.75 and 0.69 for R. palustris and R. acetosa, respectively), as well as in ethylene-treated and ethylene followed by anoxia-treated petioles (Figure 7D). These Rumex group VII ERFs also contain the characteristic N-end motif required for oxygen-dependent degradation. We hypothesize that increased transcription and translation of membrane sequestered RAP2.12 orthologs could mechanistically explain priming to anoxia by ethylene in R. palustris.
The difference in submergence survival strategy and hypoxia-responsive gene expression between R. acetosa and R. palustris indicates that ethylene plays an important role in the transition from submergence to low oxygen conditions. Interestingly, ADH1 induction during low oxygen conditions was also shown to require ethylene signaling in Arabidopsis (Peng et al., 2001). Furthermore, preceding anoxia by hypoxia has also been shown to improve anoxia tolerance in a wide range of species, including those considered tolerant and susceptible, and is generally attributed to an enhanced opportunity for adaptive protein synthesis (Bailey-Serres and Voesenek, 2008). The results of this study demonstrate that under oxygen replete conditions, ethylene treatment is sufficient to prime the frequently flooded R. palustris for anoxia, a process that is absent in the rarely flooded R. acetosa. This suggests that ethylene-induced priming to future anoxia is a trait of wetland-adapted plants that, even when programmed to escape submergence through shoot elongation, are at risk of severely impoverished oxygen conditions.
Using a genome-scale approach, we identified novel molecular and physiological players that underlie the contrasting growth strategies of R. acetosa and R. palustris. Two categories of flooding responsive transcripts were identified: those that were regulated similarly in both R. acetosa and R. palustris, and those that were regulated only in one of the two species. The overlapping transcriptomic responses of the two Rumex species were mainly related to coping with the underwater environment, whereas the species-specific transcript changes can be associated with either growth suppression or stimulation. Additional resolution of submergence-induced hormonal changes and responsiveness was further uncovered; for instance, we identified a full suite of cell wall–modifying agents and ABA-signaling players that might be able to explain variation in GA sensitivity upon submergence among species.
Important processes in the quiescent species were primarily related to changes in metabolism, especially with regard to SnRK1, nitrogen metabolism, and PCK. In R. palustris, which utilizes the flooding-escape strategy, ethylene is able to capture the signaling pathways typical of photomorphogenesis and shade avoidance via ABA downregulation. This likely represents a unique mechanism compared with underwater shoot elongation in rice. Ethylene was also shown to play a role in preparing R. palustris, but not R. acetosa, for potential future low oxygen conditions. Overall, we present a molecular and physiological explanation of the contrasting flooding survival strategies of two Rumex species. Investigating the diversity of adaptive processes to adverse environments is needed to improve crop performance in areas not suited for growing current conventional crops. Therefore, studying wild species, where these mechanisms have evolved in nature, provides a rich source of novel tolerance mechanisms, as demonstrated by this study.
METHODS
Plant Growth and Experimentation
R. acetosa and R. palustris were grown in climate-controlled walk-in growth chambers (160 μmol m−2 s−1 photosynthetically active radiation; 16-h day, 8-h night; 20°C, 70% relative humidity) and completely submerged (depth of 25 cm) in overnight acclimatized water. Ethylene and anoxia treatments were performed in humidified glass cuvettes with a continuous gas through-flow, with 5 μL L−1 ethylene-air gas mixture or pure N2. Petiole elongation was determined using linear displacement transducers (Benschop et al., 2005; Pierik et al., 2011).
RNA Isolation and Expression
RNA was extracted from the youngest petiole using the kiefer protocol (Benschop et al., 2005; Pierik et al., 2011). This was followed by DNase treatment (DNA-free; Ambion) and subsequent reverse transcription including RNase inhibitor (Invitrogen). Quantitative RT-PCR was performed using a 20-μL SYBR Green reaction mixture with gene-specific primers (see Supplemental Table 2 online) and TUBULIN as reference.
454 Sequencing and Bioinformatics
RNA for 454 sequencing was derived from the youngest petiole over a 24-h time period from two independent experiments, which were pooled in equimolar amounts. Harvest time points for R. palustris were as follows: 0h; air 4h; and submerged 1h, 4h, and 12h. Harvest time points for R. acetosa were as follows: 0 h; air 10 h; and submerged 2 h, 10 h, and 24 h. Normalized cDNA libraries were constructed by Vertis Biotechnologie and sequenced by Macrogen. De novo reference transcriptome assembly was done with Roche GS FLX software (V2.3) using a minimum overlap length of 40 nucleotides, a seed length of 16 nucleotides, a minimum percent identity of 90, and alignment and difference scores of 2 and −3, respectively. Gene family identification was done using OrthoMCL V1.4 (Enright et al., 2002; Li et al., 2003). Here Tribe Markov Clustering of the similarity matrix obtained by all-versus-all blastN (Altschul et al., 1990) on transcript sequences used an inflation factor 1.1 for plant kingdom–wide analysis and 3.0 for the direct comparison between R. acetosa and R. palustris.
Illumina Sequencing and Bioinformatics
RNA for Illumina sequencing was pooled from two independent experiments consisting of 10 replicates per treatment with 10 individual youngest petioles each. Sequencing and library construction were performed by Macrogen. Read mapping against a reference transcriptome was done using MAQ0.7.1 software (maximum mismatch of 2). Normalization, differential expression with the Benjamini-Hochberg correction, and fold changes were based on a negative binomial model of OMCL family read numbers, determined using the Bioconductor edgeR package (see Supplemental Data Set 1 online) (Robinson and Oshlack, 2010), and combined with the Limma package to implement a species × treatment model (Smyth, 2004). Common dispersion was estimated using 144 housekeeping genes based on Arabidopsis microarrays (Czechowski et al., 2005), which were assumed not to be differentially expressed. OMCL families with fewer than 50 reads in both air and submergence were excluded from analysis. A GO analysis, with the Bioconductor GOseq package incorporating gene length bias correction (Young et al., 2010), was performed using OMCL families with |logFC|>1. A BLAST score of Eval <10−10 was used for the annotation of these OMCL families
Metabolite Profiling and Enzyme Activity
Gas chromatography–mass spectrometry and ultra-performance liquid chromatography–mass spectrometry (UPLC-MS) metabolite profiling was performed as previously described (Barding et al., 2013). The metabolites 3-phosphoglycerate, 6-phosphoguconate, oxaloacetate, and α-ketoglutarate were analyzed by UPLC-MS and Suc, Glc, citrate, succinate, malate, pyroglutamate, aspartate, Gly, myo-inositol, ascorbate, shikimate, Glu, β-Ala, lactate, Gly, β-sitosterol, campesterol, tartrate, propanoate, and erythronate were analyzed by gas chromatography–mass spectrometry. For UPLC-MS measurements, weak anion exchange solid phase extraction cartridges were conditioned with methanol followed by basified water. Dried extracts were reconstituted in water, loaded onto the cartridge, washed with water and methanol, eluted with 80:20 H2O:methanol containing 5% formic acid and dried by speed vacuum. Samples were reconstituted in 200 μL water and 20 μL was injected onto a 2.1 × 100 mm Acquity HSS T3 column. Solvent A was aqueous 10 mM dibutylamine buffer pH 7.4 and solvent B was 10 mM dibutylamine buffer in methanol at a pH meter reading of 8.0. Mass spectrometry measurements used a Waters electron spray ionization quadrupole time-of-flight spectrometer operated in negative mode. Metabolite identities were confirmed through a comparison of an in-house library generated from metabolite standards.
Adenylates, amino acids, nitrate, and ammonia were identified from potassium hydroxide (0.4 M) neutralized and bicine (1 M) buffered perchloric acid (0.83 N) extracts. The subsequent pellet was used for starch and protein determination after washing three times with ethanol and solubilization with 0.2 M potassium hydroxide (90°C, 60 min). Adenylates were determined as previously described (Mustroph et al., 2006), as were ammonia (Novozamsky et al., 1974), nitrate (Cataldo et al., 1975), amino acids (Rosen, 1957), protein (Bradford, 1976), and starch (Mustroph et al., 2006). PCK activity was determined by assaying the activity in the reverse direction through monitoring the decline in NADH as previously described (Malone et al., 2007).
Respiration and Internal Oxygen Concentrations
Excised youngest petioles, one for R. acetosa and two for R. palustris per replicate, were fixed in 2 mL glass microrespiration chambers filled with tap water, which were also used for submergence experiments. A decline in O2 levels was determined with O2 microelectrodes (MicroResp; Unisense) at 20°C in darkness (Brodersen et al., 2008). Profiles of internal O2 partial pressure of the petiole were measured in light (175 µmol photons m−2 s−1) and O2 and CO2 of the submergence solution were kept at atmospheric equilibrium (20°C) by bubbling with air. A motorized micromanipulator (MC-232; Unisense) mounted with a microelectrode (tip diameter = 25 µm, OX25; Unisense) was used with a step size of 25 µm (Pedersen et al., 2006).
Accession Numbers
Sequence data from this article can be found in the Sequence Read Archive and the Transcriptome Shotgun Assembly under the following accession ranges: HAAK01000001 to HAAK01031986 (for R. acetosa) and HAAL01000001 to HAAL01049016 (R. palustris). Accession numbers along with their corresponding OMCL family identification numbers for all of the genes mentioned in this study are available in Supplemental Data Set 2 online.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Sequencing and Transcriptome Analysis of Rumex.
Supplemental Figure 2. Gene Ontology Enrichment.
Supplemental Figure 3. OMCL Families Involved in the Core Network of the Submergence Escape Response.
Supplemental Figure 4. Transcriptional Changes of Rumex OMCL Families Involved in Metabolism.
Supplemental Figure 5. Metabolites Identified by GC-MS and UPLC-MS.
Supplemental Figure 6. Effect of Far-Red Light Removal on Elongation Growth of R. palustris.
Supplemental Figure 7. The Rumex Hypoxia Core Set Orthologs.
Supplemental Table 1. Sequencing Output, Assembly, and Mapping Statistics of 454 and Illumina Sequencing.
Supplemental Table 2. Primer Pairs Used for Quantitative RT-PCR Analysis of Transcript Abundance.
Supplemental Data Set 1. Read Count, Differential Expression, and Annotation of OMCL Families.
Supplemental Data Set 2. Contig Read Count, Accession Numbers, and OMCL Family Identification Numbers.
Supplementary Material
Acknowledgments
Research in the Voesenek laboratory was funded by grants from Utrecht University, the Centre for Biosystems Genomics (CBSG2012), the Netherlands Organisation for Scientific Research (Grants 819.01.006 to L.A.C.J.V. and H.v.V; Veni 86312013 to R.S.), and the U.S. National Science Foundation (IOS-1121626 to J.B.S. and C.K.L.; DGE-0504249 fellowship to G.A.B.). The authors especially thank Ronald Leito and Frits Kindt (Utrecht University) for photography.
AUTHOR CONTRIBUTIONS
H.v.V., C.K.L., R.P., J.B.-S., L.A.C.J.V., and R.S. designed the research. H.v.V., G.A.B., M.V.-v.E., R.A.M.W.-E., O.P., E.J.W.V., and R.S. performed research. H.v.V., A.M., G.A.B., and R.S. analyzed data. H.v.V., G.A.B., R.P., J.B.-S., L.A.C.J.V., and R.S. wrote the article.
References
- Akman M., Bhikharie A.V., McLean E.H., Boonman A., Visser E.J.W., Schranz M.E., van Tienderen P.H. (2012). Wait or escape? Contrasting submergence tolerance strategies of Rorippa amphibia, Rorippa sylvestris and their hybrid. Ann. Bot. (Lond.) 109: 1263–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Baena-González E., Sheen J. (2008). Convergent energy and stress signaling. Trends Plant Sci. 13: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E., Rolland F., Thevelein J.M., Sheen J. (2007). A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J., Voesenek L.A.C.J. (2008). Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Barding G.A., Jr, Béni S., Fukao T., Bailey-Serres J., Larive C.K. (2013). Comparison of GC-MS and NMR for metabolite profiling of rice subjected to submergence stress. J. Proteome Res. 12: 898–909 [DOI] [PubMed] [Google Scholar]
- Barding G.A., Jr, Fukao T., Béni S., Bailey-Serres J., Larive C.K. (2012). Differential metabolic regulation governed by the rice SUB1A gene during submergence stress and identification of alanylglycine by 1H NMR spectroscopy. J. Proteome Res. 11: 320–330 [DOI] [PubMed] [Google Scholar]
- Benjamins R., Ampudia C.S.G., Hooykaas P.J.J., Offringa R. (2003). PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 132: 1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop J.J., Bou J., Peeters A.J.M., Wagemaker N., Gühl K., Ward D., Hedden P., Moritz T., Voesenek L.A.C.J. (2006). Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin1. Plant Physiol. 141: 1644–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop J.J., Jackson M.B., Gühl K., Vreeburg R.A.M., Croker S.J., Peeters A.J.M., Voesenek L.A.C.J. (2005). Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J. 44: 756–768 [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brodersen K.P., Pedersen O., Walker I.R., Jensen M.T. (2008). Respiration of midges (Diptera; Chironomidae) in British Columbian lakes: Oxy-regulation, temperature and their role as palaeo-indicators. Freshw. Biol. 53: 593–602 [Google Scholar]
- Brown N.J., et al. (2010). C acid decarboxylases required for C photosynthesis are active in the mid-vein of the C species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J. 61: 122–133 [DOI] [PubMed] [Google Scholar]
- Catalaldo D.A., Haroon M., Schrader L.E., Youngs V.L. (1975). Rapid colorometric determination of nitrate in plant tissue by nitration of salicyclic acid. Commun. Soil Sci. Plant Anal. 6: 71–80 [Google Scholar]
- Chen X., Pierik R., Peeters A.J.M., Poorter H., Visser E.J.W., Huber H., de Kroon H., Voesenek L.A.C.J. (2010). Endogenous abscisic acid as a key switch for natural variation in flooding-induced shoot elongation. Plant Physiol. 154: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer T.D., Voesenek L.A.C.J. (2009). Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 36: 665–681 [DOI] [PubMed] [Google Scholar]
- Cox M.C.H., Benschop J.J., Vreeburg R.A.M., Wagemaker C.A.M., Moritz T., Peeters A.J.M., Voesenek L.A.C.J. (2004). The roles of ethylene, auxin, abscisic acid, and gibberellin in the hyponastic growth of submerged Rumex palustris petioles. Plant Physiol. 136: 2948–2960, discussion 3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M.C.H., Peeters A.J.M., Voesenek L.A.C.J. (2006). The stimulating effects of ethylene and auxin on petiole elongation and on hyponastic curvature are independent processes in submerged Rumex palustris. Plant Cell Environ. 29: 282–290 [DOI] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M.K., Scheible W.-R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington, C.D., and Wylie, A.P. (1955). Chromosome Atlas of Flowering Plants. (London: Allen & Unwin). [Google Scholar]
- de Wit M., Kegge W., Evers J.B., Vergeer-van Eijk M.H., Gankema P., Voesenek L.A.C.J., Pierik R. (2012). Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proc. Natl. Acad. Sci. USA 109: 14705–14710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Alvarado A., Walker R.P., Leegood R.C. (2007). Phosphoenolpyruvate carboxykinase in developing pea seeds is associated with tissues involved in solute transport and is nitrogen-responsive. Plant Cell Environ. 30: 225–235 [DOI] [PubMed] [Google Scholar]
- Duek P.D., Elmer M.V., van Oosten V.R., Fankhauser C. (2004). The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr. Biol. 14: 2296–2301 [DOI] [PubMed] [Google Scholar]
- Ekblom R., Galindo J. (2011). Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity (Edinb) 107: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzenga, J.T.M., and van Veen, H. (2010). Waterlogging and plant nutrient uptake. In Waterlogging Signalling and Tolerance in Plants, S. Mancuso and S.N. Shabala, eds (Heidelberg: Springer Verlag), pp. 23–35. [Google Scholar]
- Enright A.J., Van Dongen S., Ouzounis C.A. (2002). An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 30: 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Ito M., Nishida I., Watanabe A. (2000). Multiple signaling pathways in gene expression during sugar starvation. Pharmacological analysis of din gene expression in suspension-cultured cells of Arabidopsis. Plant Physiol. 124: 1139–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Yoshikawa Y., Sato T., Inada N., Ito M., Nishida I., Watanabe A. (2001). Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol. Plant. 111: 345–352 [DOI] [PubMed] [Google Scholar]
- Fukao T., Xu K., Ronald P.C., Bailey-Serres J. (2006). A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18: 2021–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galstyan A., Cifuentes-Esquivel N., Bou-Torrent J., Martinez-Garcia J.F. (2011). The shade avoidance syndrome in Arabidopsis: A fundamental role for atypical basic helix-loop-helix proteins as transcriptional cofactors. Plant J. 66: 258–267 [DOI] [PubMed] [Google Scholar]
- Gibbs D.J., Lee S.C., Isa N.M., Gramuglia S., Fukao T., Bassel G.W., Correia C.S., Corbineau F., Theodoulou F.L., Bailey-Serres J., Holdsworth M.J. (2011). Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., et al. (2009). The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Hornitschek P., Lorrain S., Zoete V., Michielin O., Fankhauser C. (2009). Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 28: 3893–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun Y., Lee I. (2006). KIDARI, encoding a non-DNA binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol. Biol. 61: 283–296 [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P.H. (1988). Naturally occurring auxin transport regulators. Science 241: 346–349 [DOI] [PubMed] [Google Scholar]
- Jang I.C., Yang J.Y., Seo H.S., Chua N.H. (2005). HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 19: 593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K.H., Seo Y.S., Walia H., Cao P., Fukao T., Canlas P.E., Amonpant F., Bailey-Serres J., Ronald P.C. (2010). The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiol. 152: 1674–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Wilson R.L., Case J.B., Binder B.M. (2012). A comparative study of ethylene growth response kinetics in eudicots and monocots reveals a role for gibberellin in growth inhibition and recovery. Plant Physiol. 160: 1567–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kang J.Y., Cho D.I., Park J.H., Kim S.Y. (2004). ABF2, an ABRE-binding bZIP factor, is an essential component of glucose signaling and its overexpression affects multiple stress tolerance. Plant J. 40: 75–87 [DOI] [PubMed] [Google Scholar]
- Lee S., Lee S., Yang K.Y., Kim Y.M., Park S.Y., Kim S.Y., Soh M.S. (2006). Overexpression of PRE1 and its homologous genes activates Gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 47: 591–600 [DOI] [PubMed] [Google Scholar]
- Leegood R.C., Walker R.P. (2003). Regulation and roles of phosphoenolpyruvate carboxykinase in plants. Arch. Biochem. Biophys. 414: 204–210 [DOI] [PubMed] [Google Scholar]
- Leivar P., Tepperman J.M., Cohn M.M., Monte E., Al-Sady B., Erickson E., Quail P.H. (2012). Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24: 1398–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li G., Gao S., Martinez C., He G., Zhou Z., Huang X., Lee J.-H., Zhang H., Shen Y., Wang H., Deng X.W. (2010). Arabidopsis transcription factor ELONGATED HYPOCOTYL5 plays a role in the feedback regulation of phytochrome A signaling. Plant Cell 22: 3634–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Stoeckert C.J., Jr, Roos D.S. (2003). OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 13: 2178–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.F., Li Y.J., An Y.H., Xiong L.J., Shao X.H., Wang Y., Sun Y. (2009). AKINbeta1 is involved in the regulation of nitrogen metabolism and sugar signaling in Arabidopsis. J. Integr. Plant Biol. 51: 513–520 [DOI] [PubMed] [Google Scholar]
- Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L.A.C.J., Perata P., van Dongen J.T. (2011). Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Malone S., Chen Z.H., Bahrami A.R., Walker R.P., Gray J.E., Leegood R.C. (2007). Phosphoenolpyruvate carboxykinase in Arabidopsis: Changes in gene expression, protein and activity during vegetative and reproductive development. Plant Cell Physiol. 48: 441–450 [DOI] [PubMed] [Google Scholar]
- Mommer L., Visser E.J.W. (2005). Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann. Bot. (Lond.) 96: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L., Lenssen J.P.M., Huber H., Visser E.J.W., De Kroon H. (2006a). Ecophysiological determinants of plant performance under flooding: A comparative study of seven plant families. J. Ecol. 94: 1117–1129 [Google Scholar]
- Mommer L., Pons T.L., Visser E.J.W. (2006b). Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J. Exp. Bot. 57: 283–290 [DOI] [PubMed] [Google Scholar]
- Mommer L., Wolters-Arts M., Andersen C., Visser E.J.W., Pedersen O. (2007). Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytol. 176: 337–345 [DOI] [PubMed] [Google Scholar]
- Moons A. (2005). Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitam. Horm. 72: 155–202 [DOI] [PubMed] [Google Scholar]
- Mustroph A., Boamfa E.I., Laarhoven L.J.J., Harren F.J.M., Albrecht G., Grimm B. (2006). Organ-specific analysis of the anaerobic primary metabolism in rice and wheat seedlings. I: Dark ethanol production is dominated by the shoots. Planta 225: 103–114 [DOI] [PubMed] [Google Scholar]
- Mustroph A., Lee S.C., Oosumi T., Zanetti M.E., Yang H., Ma K., Yaghoubi-Masihi A., Fukao T., Bailey-Serres J. (2010). Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 152: 1484–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A., Zanetti M.E., Jang C.J.H., Holtan H.E., Repetti P.P., Galbraith D.W., Girke T., Bailey-Serres J. (2009). Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Hattori Y., Ashikari M. (2010). Stunt or elongate? Two opposite strategies by which rice adapts to floods. J. Plant Res. 123: 303–309 [DOI] [PubMed] [Google Scholar]
- Novozamsky I., van Eck R., van Schouwenburg J.C., Walinga I. (1974). Total nitrogen determination in plant material by means of the indolphenolblue method. Neth. J. Agric. Sci. 22: 3–5 [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Pedersen O. (1993). Long-distance water transport in aquatic plants. Plant Physiol. 103: 1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Vos H., Colmer T.D. (2006). Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ. 29: 1388–1399 [DOI] [PubMed] [Google Scholar]
- Peng H.P., Chan C.S., Shih M.C., Yang S.F. (2001). Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 126: 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R., De Wit M., Voesenek L.A.C.J. (2011). Growth-mediated stress escape: Convergence of signal transduction pathways activated upon exposure to two different environmental stresses. New Phytol. 189: 122–134 [DOI] [PubMed] [Google Scholar]
- Pierik R., Tholen D., Poorter H., Visser E.J.W., Voesenek L.A.C.J. (2006). The Janus face of ethylene: Growth inhibition and stimulation. Trends Plant Sci. 11: 176–183 [DOI] [PubMed] [Google Scholar]
- Pierik R., van Aken J.M., Voesenek L.A.C.J. (2009). Is elongation-induced leaf emergence beneficial for submerged Rumex species? Ann. Bot. (Lond.) 103: 353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders J.G.H.M., Yang Y.Y., Kamiya Y., Takahashi N., Barendse G.W.M., Blom C.W.P.M., Voesenek L.A.C.J. (1997). Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203: 20–25 [Google Scholar]
- Robinson M.D., Oshlack A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. (1957). A modified ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. 67: 10–15 [DOI] [PubMed] [Google Scholar]
- Santner A.A., Watson J.C. (2006). The WAG1 and WAG2 protein kinases negatively regulate root waving in Arabidopsis. Plant J. 45: 752–764 [DOI] [PubMed] [Google Scholar]
- Sasidharan R., Voesenek L.A., Pierik R. (2011). Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 30: 548–562 [Google Scholar]
- Setter T.L., Laureles E.V. (1996). The beneficial effect of reduced elongation growth on submergence tolerance in rice. J. Exp. Bot. 47: 1551–1559 [Google Scholar]
- Shabala S. (2011). Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 190: 289–298 [DOI] [PubMed] [Google Scholar]
- Silvertown J., Dodd M.E., Gowing D.J.G., Mountford J.O. (1999). Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 400: 61–63 [Google Scholar]
- Smyth G.K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3: e3. [DOI] [PubMed] [Google Scholar]
- Steindler C., Matteucci A., Sessa G., Weimar T., Ohgishi M., Aoyama T., Morelli G., Ruberti I. (1999). Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- van Eck W.H.J.M., van de Steeg H.M., Blom C.W.P.M., de Kroon H. (2004). Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos 107: 393–405 [Google Scholar]
- Vervuren P.J.A., Blom C.W.P.M., de Kroon H. (2003). Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. J. Ecol. 91: 135–146 [Google Scholar]
- Voesenek L.A.C.J., Bailey-Serres J. (2009). Plant biology: Genetics of high-rise rice. Nature 460: 959–960 [DOI] [PubMed] [Google Scholar]
- Voesenek L.A.C.J., Sasidharan R. (2013). Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol (Stuttg) 15: 426–435 [DOI] [PubMed] [Google Scholar]
- Voesenek L.A.C.J., Banga M., Thier R.H., Mudde C.M., Harren F.J.M., Barendse G.W.M., Blom C.W.P.M. (1993a). Submergence-induced ethylene synthesis, entrapment, and growth in two plant species with contrasting flooding resistances. Plant Physiol. 103: 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek L.A.C.J., Rijnders J.H.G.M., Peeters A.J.M., van de Steeg H.M., de Kroon H. (2004). Plant hormones regulate fast shoot elongation under water: From genes to communities. Ecology 85: 16–27 [Google Scholar]
- Voesenek L.A.C.J., Van Oorschot F.J.M.M., Smits A.J.M., Blom C.W.P.M. (1993b). The role of flooding resistance in the establishment of Rumex seedlings in river flood plains. Funct. Ecol. 7: 105–114 [Google Scholar]
- Vreeburg R.A.M., Benschop J.J., Peeters A.J.M., Colmer T.D., Ammerlaan A.H.M., Staal M., Elzenga T.M., Staals R.H.J., Darley C.P., McQueen-Mason S.J., Voesenek L.A.C.J. (2005). Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J. 43: 597–610 [DOI] [PubMed] [Google Scholar]
- Vriezen W.H., Hulzink R., Mariani C., Voesenek L.A.C.J. (1999). 1-aminocyclopropane-1-carboxylate oxidase activity limits ethylene biosynthesis in Rumex palustris during submergence. Plant Physiol. 121: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hua D., He J., Duan Y., Chen Z., Hong X., Gong Z. (2011). Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 7: e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R.E., Ainley W.M., Nagao R.T., Conner T.W., Key J.L. (1993). Expression of the Arabidopsis AtAux2-11 auxin-responsive gene in transgenic plants. Plant Mol. Biol. 22: 731–749 [DOI] [PubMed] [Google Scholar]
- Xu K., Xu X., Fukao T., Canlas P., Maghirang-Rodriguez R., Heuer S., Ismail A.M., Bailey-Serres J., Ronald P.C., Mackill D.J. (2006). Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Yang J., Lin R., Sullivan J., Hoecker U., Liu B., Xu L., Deng X.W., Wang H. (2005). Light regulates COP1-mediated degradation of HFR1, a transcription factor essential for light signaling in Arabidopsis. Plant Cell 17: 804–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. (2010). Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 11: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Primavesi L.F., Jhurreea D., Andralojc P.J., Mitchell R.A.C., Powers S.J., Schluepmann H., Delatte T., Wingler A., Paul M.J. (2009). Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.