Down-regulation of a d-limonene synthase gene in orange fruit triggers defense cascades linked to isoprenoid metabolism and resistance to necrotroph pathogens.
Abstract
Terpenoid volatiles are isoprene compounds that are emitted by plants to communicate with the environment. In addition to their function in repelling herbivores and attracting carnivorous predators in green tissues, the presumed primary function of terpenoid volatiles released from mature fruits is the attraction of seed-dispersing animals. Mature oranges (Citrus sinensis) primarily accumulate terpenes in peel oil glands, with d-limonene accounting for approximately 97% of the total volatile terpenes. In a previous report, we showed that down-regulation of a d-limonene synthase gene alters monoterpene levels in orange antisense (AS) fruits, leading to resistance against Penicillium digitatum infection. A global gene expression analysis of AS versus empty vector (EV) transgenic fruits revealed that the down-regulation of d-limonene up-regulated genes involved in the innate immune response. Basal levels of jasmonic acid were substantially higher in the EV compared with AS oranges. Upon fungal challenge, salicylic acid levels were triggered in EV samples, while jasmonic acid metabolism and signaling were drastically increased in AS orange peels. In nature, d-limonene levels increase in orange fruit once the seeds are fully viable. The inverse correlation between the increase in d-limonene content and the decrease in the defense response suggests that d-limonene promotes infection by microorganisms that are likely involved in facilitating access to the pulp for seed-dispersing frugivores.
Plants are sessile organisms that produce and emit a vast array of volatile organic compounds (VOCs) to communicate between parts of the same plant and with other plants. It is generally accepted that the original role of these compounds in nature is related to defense functions (Degenhardt et al., 2003). Most VOCs are terpenoids, fatty acid degradation compounds, phenylpropanoids, and amino acid-derived products. Among these, terpenoids are likely to be the most abundant and expensive to produce (Gershenzon, 1994). Terpenoids are isoprenoid-derived compounds synthesized through the condensation of C5 isoprene units, a process that is catalyzed by a wide diversity of terpene synthases using geranyl diphosphate (GDP), farnesyl diphosphate (FDP), and geranylgeranyl diphosphate (GGDP) as substrates. These reactions give rise to the C5 hemiterpenes, the C10 monoterpenes, the C15 sesquiterpenes, and the C20 diterpenes (Dudareva et al., 2006).
In green tissues, volatile terpenoid synthesis is either induced upon wounding or occurs constitutively; terpenes can be then stored in specific organs or tissues where they would be most effective in defense responses, such as leaf trichomes, resin ducts and lacticifers, pockets near the epidermis, or secretory cavities in Citrus spp. (Langenheim, 1994; Turner et al., 2000; Trapp and Croteau, 2001; Voo et al., 2012). Genetic engineering experiments have demonstrated that specific terpenoid compounds emitted by leaves can intoxicate, repel, or deter herbivores (Aharoni et al., 2003; Wu et al., 2006), or they may attract the natural predators and parasitoids of damaging herbivores to protect plants from further damage (Kappers et al., 2005; Schnee et al., 2006). These terpenoids are naturally found in complex mixtures, and it has been proposed that they can act synergistically, as in conifer resin, for simultaneous protection against pests and pathogens (Phillips and Croteau, 1999). Although fatty acid degradation products (such as jasmonates) and phenylpropanoids (such as salicylates) as well as their volatile and nonvolatile precursors are clearly involved in many induced defense responses against pests and pathogens (Glazebrook, 2005), much less is known regarding the participation of terpenoid volatiles in the defense against microorganisms in plants and about the possible interactions of these terpenoids with phytohormones.
In contrast to their function in leaves, when released from flowers and mature fruits, the main function of terpenoid volatiles is in the attraction of pollinators (Pichersky and Gershenzon, 2002; Kessler et al., 2008; Junker and Blüthgen, 2010; Schiestl, 2010) and seed-dispersing animals (Lomáscolo et al., 2010; Rodríguez et al., 2011b), respectively. Fruit maturation and ripening are usually associated with large increases in the synthesis and accumulation of specific flavored volatiles, which are proposed to function as signals for seed dispersal (Auldridge et al., 2006; Goff and Klee, 2006; Rodríguez et al., 2013).
Upon wounding, plant responses to biotic stresses are orchestrated locally and systemically by signaling molecules. Among these molecules, the jasmonates regulate defenses against arthropod herbivores and necrotroph fungal pathogens as well as biotrophic pathogens, such as some mildews (Ellis and Turner, 2001; Stintzi et al., 2001; Kessler et al., 2004; Li et al., 2005; Wasternack, 2007; Browse and Howe, 2008). In addition to jasmonates, molecules such as salicylic acid (SA) and ethylene appear to regulate distinct defense pathways and are major synergistic (Mur et al., 2006) or antagonistic (De Vos et al., 2005) regulators of plant innate immunity. Plants produce a specific blend of these alarm signals after pathogen or pest attacks, and the production of these molecules varies greatly in quantity, composition, and timing. These signals activate differential sets of defense-related genes that eventually determine the nature of the defense response against the attacker (Reymond and Farmer, 1998; Rojo et al., 2003; De Vos et al., 2005). All genes that encode enzymes involved in the biosynthesis of jasmonates are jasmonic acid (JA) inducible (Wasternack, 2006), indicating that JA biosynthesis is regulated by positive feedback. The precursor for the biosynthesis of JA is α-linolenic acid. The activity of the 13-lipoxygenase (LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC) enzymes converts α-linolenic acid to cis-(+)-12-oxophytodienoic acid (OPDA). OPDA REDUCTASE3 catalyzes the reduction of OPDA (and dinor-OPDA) to oxo-pentenyl-cycloheptane-octanoic acid, which, in turn, undergoes three rounds of β-oxidation leading to jasmonyl-CoA formation. Jasmonyl-CoA is then cleaved by a putative thioesterase yielding (+)-7-iso-JA, which equilibrates to the more stable (−)-JA (Wasternack and Kombrink, 2010).
The exogenous application of jasmonates on plants and the existence of mutant and/or transgenic plants altered in JA biosynthesis or signaling have led to altered susceptibility or resistance to pathogens. Impaired JA biosynthesis or signaling is generally associated with decreased levels of defensive compounds, including VOCs, and reduced plant biomass and/or fitness under insect attack (Howe et al., 1996; Halitschke and Baldwin, 2004). For example, Arabidopsis (Arabidopsis thaliana) mutants defective in JA perception (e.g. coronatine-insensitive1 [coi1]) or biosynthesis (e.g. aos and defective in anther dehiscence1) are susceptible to pathogen infections (Feys et al., 1994; Xie et al., 1998; Park et al., 2002; Turner et al., 2002). In contrast, mutants (e.g. constitutive expression of vegetative storage protein1 and Arabidopsis Ser/Thr phosphatase of type 2C1) with constitutive or wound-induced activation of the JA pathway exhibit enhanced resistance to fungal pathogens and pests and phenotypes characteristic of JA-treated plants (Ellis and Turner, 2001; Ellis et al., 2002; Schweighofer et al., 2007).
Sweet orange (Citrus sinensis) is a perennial tree species that is exposed to recurrent biotic and abiotic challenges during its decades of growth in orchards. Orange fruits undergo a nonclimacteric maturation process in which the biochemistry, physiology, and structure of the organ are altered to complete the release of mature seeds. These changes typically include fruit growth and texture modification; color change through the degradation of chlorophylls and a parallel induction of carotenogenesis in the peel (flavedo) and pulp; flavonoid accumulation in the pulp; increases and decreases in the sugar and acid contents, respectively; and global accumulation and selective emission of volatile terpenoids (Spiegel-Roy and Goldschmidt, 1996). In nature, d-limonene accumulates gradually in the oil glands of the peel during fruit development and reaches its maximum level shortly before the breaker stage, followed by a steady decline during maturation (Attaway et al., 1967; Kekelidze et al., 1989; Rodríguez et al., 2011b). The high amount of d-limonene that accumulates in orange peels has a tremendous metabolic cost, suggesting an important biological role for this terpene and other related compounds in the interactions between fruits and the biotic environment.
Previously, we examined the biological role of d-limonene by manipulating oil gland chemistry via the antisense (AS) overexpression of a d-limonene synthase gene from Satsuma mandarin (Citrus unshiu) in orange fruits. Compared with empty vector (EV) controls, fruit peels from AS transformants showed a dramatic reduction in d-limonene accumulation; decreased levels of other monoterpenes, sesquiterpenes, and monoterpene aldehydes; and increased levels of monoterpene alcohols. When challenged with the necrotroph fungus Penicillium digitatum, the causal agent of green mold rot, AS-transformed fruits were highly resistant to fungal infection. Full susceptibility to P. digitatum infection was restored when AS fruits were supplemented with d-limonene but not other monoterpene alcohols, indicating that d-limonene accumulation in the orange peel was required for the successful progress of this plant-pathogen interaction (Rodríguez et al., 2011a, 2011b). Green mold rot is the most important postharvest disease of citrus fruit worldwide, accounting for up to 60% to 80% of total losses during postharvest life of the fruit. P. digitatum is considered to be a specialist pathogen of citrus fruits that efficiently infects the peel through injuries in which ubiquitous fungal spores germinate and rapidly colonize the surrounding areas (Droby et al., 2008). The control of this pathogen relies heavily on the use of synthetic chemicals, but concerns regarding their potential negative effects on human health and also the generation of fungicide-resistant strains have encouraged finding alternatives, such as the generation of citrus trees with fruits that are genetically resistant to the pathogen.
In this work, to better understand the mechanism underlying the constitutive resistance to P. digitatum conferred by the reduction of limonene in AS orange fruits, we analyzed the pattern of fruit growth and the morphological and biochemical developmental characteristics and performed a global analysis of gene expression using a 20K citrus microarray. The study is supplemented by examining the possible involvement of key hormone signals and isoprenoid precursors in the fruit peel. We report here that the reduced level of d-limonene in AS fruits is tightly associated with the constitutive activation of defense response signaling cascades. Our results establish, to our knowledge for the first time, a correlation between increased volatile terpene content and the decline of defense responses in a fleshy fruit during maturation, which would facilitate necrotroph fungal infections in citrus fruits.
RESULTS
Down-Regulation of a d-Limonene Synthase Gene Leads to Fungal Resistance in the Flavedo of Transgenic Orange Fruits
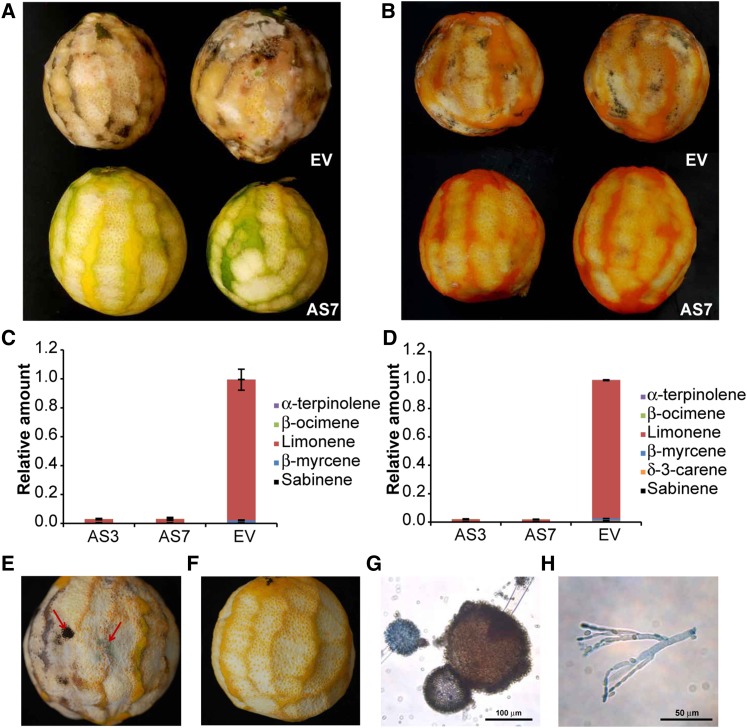
It is generally accepted that the flavedo of citrus fruit is the entrance for fungal colonization and offers higher resistance to P. digitatum infection than the albedo (inner white area without oil glands; Kavanagh and Wood, 1967; Ballester et al., 2006). To examine the contribution of flavedo terpenes and the albedo to the susceptibility of orange fruit to infection, the flavedo of orange fruits was partially peeled off and the remaining fruit was left on the bench at room temperature to facilitate the germination of ubiquitous fungal spores. Whereas EV control fruits were infected by several fungi by day 3 after peeling, samples from AS lines (AS3 and AS7) became infected to a much lower extent by day 7. This experiment was repeated monthly from August to December over two consecutive fruiting seasons with identical results that were independent of the developmental stage of the fruit (Supplemental Fig. S1) and the orange cultivar tested (e.g. cv Navelina [Fig. 1] and cv Pineapple [data not shown]). During the first week after peeling, fungal infection was exclusively restricted to wounded flavedo areas (Fig. 1, A and B), and resistance was linked to very low d-limonene levels in the oil glands of AS transformants (Fig. 1, C and D; Supplemental Table S1). The infecting fungi were morphologically identified as P. digitatum, Penicillium italicum, and Aspergillus spp (Fig. 1, E–H). Therefore, d-limonene and related terpenes produced in the flavedo of EV control fruits appeared to act as the primary inducers of fungal germination and growth (Rodríguez et al., 2011b).
Figure 1.
Fungal infection in partially peeled d-limonene independent transgenic AS and EV control cv Navelina fruits. A and B, Disease incidence at 5 d after peeling in EV lines compared with line AS7 in green (A) and mature (B) fruits. The same results were observed for line AS3. C and D, The relative amount of individual terpenes is presented as a percentage area (given as a fraction of unity) of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of 1 in green (C) and mature (D) flavedo. Data represent means ± se and are derived from at least five fruits per plant. E and F, Magnification of fruits in EV (E) and AS7 (F) lines. The red arrows indicate flavedo-infected zones of EV fruit. G and H, Microscopic identification of fungi-infected fruits. Images shown are light micrographs of Aspergillus spp (G) at 20× magnification and Penicillium spp (H) at 40× magnification. Bars = 100 μm and 50 μm, respectively.
Morphological and Biochemical Characteristics of the Orange Fruit Flavedo Are Not Altered in Transformants Showing Constitutive Down-Regulation of the d-Limonene Synthase Gene
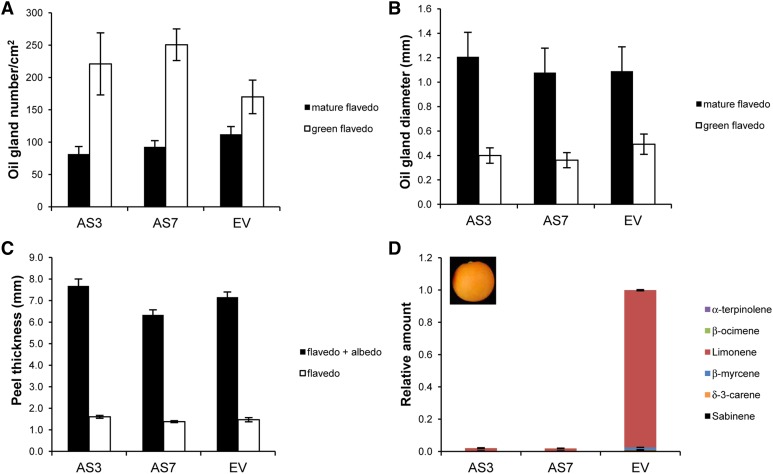
d-Limonene accounts for approximately 97% of the total terpenes in oil glands from the flavedo of orange fruit (Dugo and Di Giacomo, 2002; Rodríguez et al., 2011b). To assess whether changes in d-limonene and other monoterpenoid and sesquiterpenoid accumulation in AS versus EV transgenic fruits could have affected peel morphology, the number and size of oil glands in green and mature flavedo from transgenic cv Navelina and Pineapple oranges were determined. As shown in Figure 2, A and B, as well as in Supplemental Figure S2, A and B, oil glands increased in size as fruit grew, but they were comparable in number and diameter in AS and EV fruits. Moreover, peel thickness was also similar between AS and EV samples at the different developmental stages that were analyzed (Fig. 2C; Supplemental Fig. S2C).
Figure 2.
Characteristics of green (70 mm diameter) and mature (90 mm diameter) flavedo in AS and control EV cv Navelina sweet orange plants. A and B, Secretory oil gland number and size in green and mature flavedo. C, Peel thickness in mature fruits. Data represent means ± se and are derived from 10 fruits per plant. No significant differences were found at P < 0.05 using Fisher’s protected lsd test at each stage. D, The relative amount of individual terpenes is presented as a percentage area (given as a fraction of unity) of each terpene with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of 1 in the mature flavedo. Data represent means ± se and are derived from at least five fruits per plant.
We then tested whether the transgenic manipulation of monoterpene biosynthesis in fruits may have induced a metabolic diversion and affected the levels of other related isoprenoids that share common precursors (Supplemental Fig. S3), particularly those important during the development of orange fruit, such as chlorophylls or carotenoids. The degreening of the fruits followed the same pattern in AS and EV control lines (Supplemental Fig. S1). Chlorophyll and total carotenoid contents in EV control green and mature flavedo from cv Navelina and Pineapple oranges were similar to those found in AS lines (Fig. 3; Supplemental Fig. S4). Additionally, the percentage of individual xanthophylls and carotenes remained at nearly the same level in both EV and AS lines (Supplemental Fig. S5). Taken together, these results confirmed that fruit growth and development were not substantially altered by the drastic changes in monoterpene accumulation; thus, other factors must be responsible for the increased disease resistance found in the peel of d-limonene AS plants.
Figure 3.
Total chlorophyll and carotenoid contents in green (A and B) and mature (B) flavedo of cv Navelina sweet orange fruits. No chlorophyll was detected in the flavedo of mature fruits. Data represent means ± se and are derived from at least 10 fruits per plant. No significant differences were found at P < 0.05 using Fisher’s protected lsd test in each stage. FW, Fresh weight.
d-Limonene Down-Regulation Induces the Expression of Genes Involved in the Innate Immune Response against Pathogens
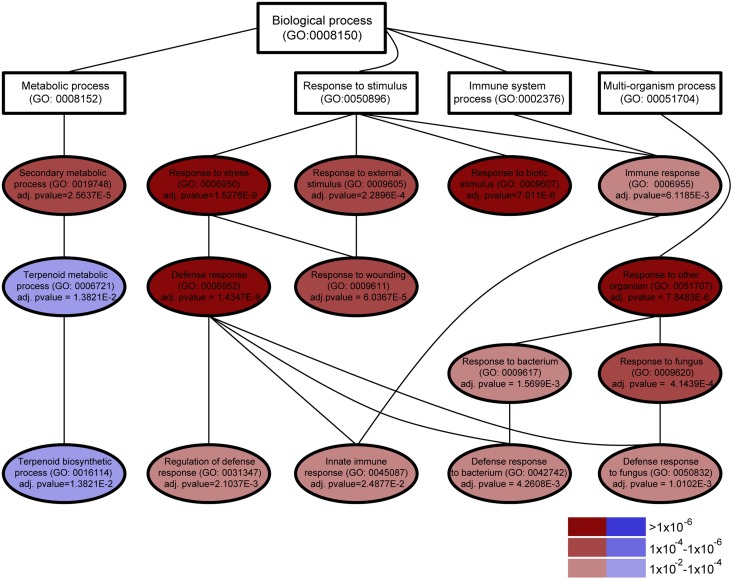
To understand the mechanisms underlying the induced resistance of AS orange fruits to P. digitatum and other fungi, large-scale gene expression analysis was carried out using a 20K citrus complementary DNA (cDNA) microarray (Martinez-Godoy et al., 2008). Using intact mature flavedo tissue, gene expression in the AS3 and AS7 lines was compared with that of two independent EV control lines. The ectopic up-regulation or down-regulation of genes involved in eliciting defense responses against herbivores and plant pests usually results in phenotypic aberrations, because such genes are also important for growth and development (Bedon et al., 2010; Kallenbach et al., 2010; Yang et al., 2012). As AS orange plants and fruits were visually indistinguishable from EV controls, we hypothesized that the impact of d-limonene synthase down-regulation on the general transcript profile would not be very high quantitatively. Then, common genes from both AS lines showing at least a 1.6-fold expression change versus EV lines were identified as differentially expressed (Tables I and II). We found differential gene expression in the AS lines, with 82.9% and 93% of genes up-regulated in AS3 and AS7, respectively (Supplemental Table S2). To elucidate key processes that were altered in AS fruits, functional enrichment categories were searched for the full robust set of differentially expressed genes (Fig. 4). Based on Gene Ontology (GO) terms, the genes down-regulated in the flavedo of AS fruits were primarily involved in biological processes associated with secondary metabolism (Fig. 4; Supplemental Fig. S6). AS down-regulation of the d-limonene synthase gene was found to reduce the transcription of nine genes, of which four encode enzymes that would be required for volatile terpenoid biosynthesis, such as a monoterpene (R)-limonene synthase gene and a putative germacrene-d synthase gene whose expression was reduced more than 4-fold in the AS versus EV samples in the microarray analysis (Fig. 5A; Table II) and at least 9- and 5-fold in quantitative reverse transcription (qRT)-PCR analyses in the AS lines (Supplemental Fig. S7).
Table I. Common differentially expressed up-regulated genes in the intact mature flavedo of two independent transgenic AS cv Navelina sweet orange plants (versus EV plants).
| Description | Citrus Unigene | AS3 Fold Change | AS7 Fold Change | Most Similar Ath Gene |
|---|---|---|---|---|
| Defense response | ||||
| Cyclic nucleotide-regulated ion channel (CNGC2) | aC32102F03EF_c | 2.03 | 1.88 | AT5G15410 |
| Cyclic nucleotide-regulated ion channel (CNGC2) | aCL5832Contig1 | 3.60 | 2.90 | AT5G15410 |
| Disease resistance protein (NBS-LRR class) | aCL5233Contig1 | 1.75 | 2.12 | AT3G14460 |
| Yellow leaf-specific gene9 (YLS9)/harpin-induced1 family protein | aCL2389Contig2 | 2.06 | 1.63 | AT2G35980 |
| Nonspecific lipid transfer protein1 (LTP1) | aCL4Contig13 | 3.31 | 3.46 | AT2G38540 |
| Phe ammonia-lyase1 (PAL1) | aCL1166Contig2 | 3.47 | 1.96 | AT2G37040 |
| Protein phosphatase 2C, putative (PP2C) | aCL683Contig1 | 2.33 | 3.41 | AT2G30020 |
| Similar to zinc finger (CCCH-type) family protein (CZF1) | aC31603G11EF_c | 2.38 | 3.53 | AT2G40140 |
| Chalcone synthase/naringenin-chalcone synthase | aCL27Contig2 | 1.85 | 1.94 | AT5G13930 |
| Encodes a member of the ERF subfamily B-3 of ERF/AP2 (ATERF-6) | aCL337Contig1 | 1.63 | 3.43 | AT4G17490 |
| R2R3-MYB family transcription factor (MYB73) | aCL693Contig1 | 3.18 | 2.34 | AT4G37260 |
| No apical meristem (NAM) family protein (NAC72) | aCL35Contig5 | 2.02 | 1.99 | AT4G27410 |
| Sodium-inducible calcium-binding protein (ACP1) | aCL1345Contig2 | 1.75 | 3.97 | AT5G49480 |
| Vacuolar processing enzyme γ | aCL554Contig1 | 1.62 | 2.11 | AT4G32940 |
| Peptidase U7 family protein (SPPA) | aCL27Contig1 | 1.84 | 1.66 | AT1G73990 |
| Putative Ser/Thr kinase SRK2F | aC04002A03SK_c | 1.61 | 1.76 | AT4G40010 |
| Ser-Thr protein kinase | aCL5546Contig1 | 1.62 | 1.75 | AT2G40270 |
| Cellular component organization and biogenesis | ||||
| Eukaryotic translation initiation factor SUI1 | aCL1184Contig4 | 1.99 | 1.78 | AT5G54940 |
| FAD-binding domain-containing protein | aCL246Contig1 | 4.10 | 2.43 | AT2G34790 |
| Heavy metal-associated domain-containing protein | aCL2730Contig1 | 2.21 | 1.67 | AT4G08570 |
| Histone H1-3 (HIS1-3) | aCL517Contig2 | 2.62 | 1.69 | AT2G18050 |
| Peptidase S41 family protein similar to PSII D1 protein-processing enzyme | aCL7817Contig1 | 1.87 | 2.02 | AT3G57680 |
| Cellulose synthase family protein | aCL1355Contig1 | 1.73 | 1.82 | AT2G32540 |
| Other | ||||
| CCR4-associated factor | aCL206Contig1 | 3.26 | 3.61 | AT5G22250 |
| Strictosidine synthase family protein | aC31201B02EF_c | 2.09 | 1.90 | AT3G59530 |
| WD-40 repeat family protein | aCL6446Contig1 | 2.19 | 1.90 | AT1G53090 |
| DC1 domain-containing protein | aCL2160Contig1 | 1.71 | 1.78 | AT1G60420 |
| Copper chaperone (CCH) related | aCL4708Contig1 | 1.68 | 1.90 | AT5G63530 |
| Unknown | ||||
| 3-Oxo-5-α-steroid 4-dehydrogenase family protein | aC01011F03SK_c | 2.14 | 2.26 | AT5G16010 |
| Calcium-binding protein (CML4) | aCL7914Contig1 | 2.09 | 1.71 | AT1G21550 |
| Calmodulin | aCL535Contig3 | 2.85 | 2.93 | AT3G10190 |
| Calcium-binding EF hand family protein | aCL8972Contig1 | 1.67 | 1.81 | AT1G05150 |
| Chac-like family protein | aCL283Contig1 | 1.71 | 1.91 | AT4G31290 |
| Chac-like family protein | aC05802B02SK_c | 1.87 | 2.79 | AT4G31290 |
| Esterase/lipase/thioesterase family protein | aCL5939Contig1 | 2.07 | 1.75 | AT1G54570 |
| Expressed protein | aC08031A08SK_c | 2.03 | 2.08 | AT5G41110 |
| Expressed protein | aCL8468Contig1 | 1.87 | 2.13 | AT1G69760 |
| Expressed protein | aCL6840Contig1 | 2.68 | 2.26 | AT3G52740 |
| UDP-glucoronosyl/UDP glucosyl transferase family protein | aC02002E10SK_c | 1.87 | 2.04 | AT3G02100 |
| UDP-glucoronosyl/UDP-glucosyl transferase family protein | aCL5570Contig1 | 2.85 | 1.95 | AT2G36970 |
| Remorin-like protein | aCL1490Contig1 | 1.82 | 1.69 | AT2G41870 |
| ATP-sulfurylase1 (APS1) | aCL438Contig2 | 1.73 | 1.70 | AT3G22890 |
| No similar protein found | aCL8681Contig1 | 5.08 | 2.57 | |
| No similar protein found | aC08007E01SK_c | 4.64 | 9.66 | |
| No similar protein found | aCL50Contig2 | 3.38 | 2.56 | |
| No similar protein found | aCL1714Contig1 | 2.44 | 3.83 | |
| No similar protein found | aC03007D01SK_c | 2.39 | 1.87 | |
| No similar protein found | aCL101Contig2 | 2.28 | 1.65 | |
| No similar protein found | aC08007C02SK_c | 2.24 | 1.99 | |
| No similar protein found | aC31006C04EF_c | 2.13 | 1.84 | |
| No similar protein found | aC31206E07EF_c | 2.08 | 1.86 | |
| No similar protein found | aCL4787Contig1 | 2.06 | 1.83 | |
| No similar protein found | aC31807H02EF_c | 2.03 | 2.09 | |
| No similar protein found | aC31305G08EF_c | 1.97 | 1.97 | |
| No similar protein found | aC19003E02T7_c | 1.90 | 1.82 | |
| No similar protein found | aCL2819Contig1 | 1.85 | 2.80 | |
| No similar protein found | aC18001E11Rv_c | 1.66 | 1.94 | |
| No similar protein found | aC19006C07T7_c | 1.61 | 2.62 | |
Table II. Common differentially expressed down-regulated genes in the intact mature flavedo of two independent transgenic AS cv Navelina sweet orange plants (versus EV plants).
| Description | Citrus Unigene | AS3 Fold Change | AS7 Fold Change | Most Similar Ath Gene |
|---|---|---|---|---|
| Secondary metabolic process | ||||
| ATTPS-CIN; encodes the monoterpene 1,8-cineole synthase; highly similar to (R)-limonene synthase 1 | aC02013A08SK_c | −4.65 | −4.68 | AT3G25820 |
| ATTPS-CIN; encodes the monoterpene 1,8-cineole synthase; highly similar to (R)-limonene synthase 1 | aCL2450Contig1 | −3.48 | −3.12 | AT3G25820 |
| Encodes a sesquiterpene synthase (germacrene-d synthase) | aCL4874Contig1 | −4.03 | −5.48 | AT5G23960 |
| Flavonol 3-O-methyltransferase1/caffeic acid | aCL38Contig8 | −3.01 | −2.41 | AT5G54160 |
| Cellular component organization and biogenesis | ||||
| TIP1;3; major intrinsic family protein/MIP family protein; aquaporin | aCL824Contig2 | −1.94 | −2.40 | AT4G01470 |
| Unknown | ||||
| Alcohol dehydrogenase | aC34109F01EF_c | −2.27 | −2.20 | AT5G42250 |
| O-Methyltransferase family 2 protein, similar to caffeic acid O-methyltransferase | aCL3052Contig1 | −1.82 | −2.27 | AT4G35160 |
| O-Methyltransferase family 2 protein, similar to caffeic acid O-methyltransferase | aCL4905Contig1 | −1.72 | −2.32 | AT4G35160 |
| F4H5.19 protein | aC20001E01SK_c | −3.55 | −3.13 | AT1G06720 |
Figure 4.
Hierarchical view of GO biological categories significantly deregulated in the intact flavedo of AS7 plants compared with EV control plants. AS down-regulation of the d-limonene synthase gene causes the down-regulation of genes required for monoterpenoid biosynthesis and the up-regulation of genes related to different defense responses in plants. Significant categories (adjusted P < 0.05) are shown using color scaling according to their significance level and to up-regulation (red) or down-regulation (blue). Other categories required to complete the hierarchy are shown in white. A similar graph was obtained for AS3 plants.
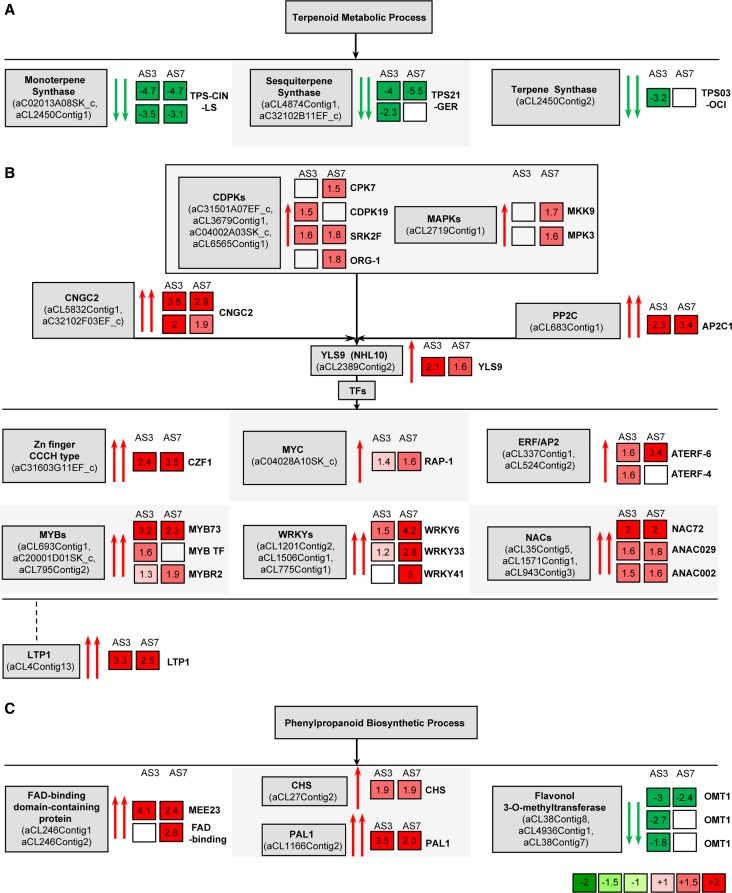
Figure 5.
Graphical representation of the genes that were differentially expressed in the flavedo of intact oranges. A, Genes involved in the terpenoid metabolic process that were down-regulated in transgenic orange plants. B, Defense-related genes that were up-regulated in transgenic orange plants. C, Genes involved in the phenylpropanoid biosynthetic process that were misregulated in transgenic orange plants. Numbers in squares indicate the ratio of expression in AS fruits compared with EV fruits. Blank squares indicate genes not detected. The most similar Arabidopsis gene functions are listed at the right side of the squares. Genes are as follows: TPS-CIN-LS, monoterpene synthase similar to limonene synthase; TPS21-GER, sesquiterpene synthase similar to germacrene-d synthase; TPS03-OCI, terpene synthase similar to β-ocimene/α-farnesene synthase; CDPKs, CPK7, CDPK19, SRK2F, and ORG-1; MAPKs, MKK9 and MPK3; CNGC2, cyclic nucleotide-regulated ion channel; PP2C, protein phosphatase 2C (AP2C1); YLS9; Zn (zinc) finger CCCH type family protein, CZF1; MYC, basic helix-loop-helix protein RAP-1; ERF/AP2, ethylene response factor/AP2 domains (ATERF6 and ATERF4); MYB, members of the MYB family TF (MYB73, MYB TF, and MYBR2); WRKY, members of the WRKY family TF (WRKY6, WRKY33, and WRKY41); NAC, NAC72, ANAC029, and ANAC002; LTP1; FAD, FAD-binding proteins (MEE23 and FAD-binding); CHS, chalcone synthase; PAL1; and flavonol 3-O-methyltransferase (OMT1).
Conversely, the biological processes that were overrepresented in the AS flavedo compared with the EV controls were primarily associated with defense responses against biotic stresses, including defense responses to fungus and bacteria and the response to wounding (Fig. 4; Supplemental Fig. S6). Fatiscan analysis allowed us to perceive a clear connection between the down-regulation of secondary metabolism and the up-regulation of the response to biotic stresses at a global level. At least half of the 58 up-regulated genes in both AS lines were related to defense. Most of the other up-regulated genes had unknown functions or did not match any known protein-coding gene in the databases. Increases in cytoplasmic calcium mediated by calcium influx are critical for triggering defense pathways in plant cells (Nicaise et al., 2009). The expression of two cyclic nucleotide-regulated ion channel genes that are likely to be involved in cellular calcium entry was two to three times higher in the AS3 and AS7 lines than in the EV lines (Fig. 5B; Table I). Several genes coding for calcium-binding proteins, including at least one calmodulin, were also up-regulated in the AS lines (Table I). This calmodulin-like protein gene was confirmed to be up-regulated by approximately 2-fold in further qRT-PCR analysis (Supplemental Fig. S7). Calcium signals are sensed by calcium-dependent protein kinases (CDPKs). Together with mitogen-activated protein kinases (MAPKs), CDPKs are essential elements for reprogramming transcriptional cascades that underlie the immune response in plants and animals (Akira et al., 2006; Boudsocq et al., 2010). Putative CDPK genes, such as homologs of CDPK19 or CPK7, and MAPK genes, such as homologs of MPK3 or MKK9, were found to be slightly up-regulated (more than 1.5-fold) in one of the AS lines (Fig. 5B; Supplemental Table S2). The citrus homolog of the early response YELLOW LEAF SPECIFIC GENE9 (YLS9) gene (also known as NON-RACE-SPECIFIC DISEASE RESISTANCE1/HARPIN-INDUCED1-LIKE10; Boudsocq et al., 2010) was found to be up-regulated approximately 2-fold in AS3 and AS7 fruits (Fig. 5B; Table I). It has also been shown that several CPKs strongly induce YLS9 (Boudsocq et al., 2010). Our results indicate that defense cascades were activated in terpene down-regulated orange fruits. Moreover, a putative protein phosphatase 2C gene that directly regulates several MAPKs (Schweighofer et al., 2007) was found to be strongly induced (by 3-fold) in both AS lines (Fig. 5B; Tables I and II).
The target genes of these signaling cascades include transcription factors (TFs) belonging to zinc finger (C-×8-C-×5-C-×3-H [CCCH]-type), myelocytomatosis (MYC), ETHYLENE RESPONSE FACTOR (ERF)/APETALA2 (AP2), myeloblastosis (MYB), WRKY (family of transcription factors comprising the highly conserved WRKYGQK peptide sequence and a zinc finger motif [CX4\x{2013}7CX22\x{2013}23HXH/C]), and NAC (for NO APICAL MERISTEM, ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR, and CUP-SHAPED COTYLEDON) family transcription factors, which have been associated with a suite of diverse mechanisms leading to defense responses (Fujita et al., 2006) and were found to be up-regulated in both AS lines (Fig. 5B; Table I; Supplemental Table S2). Citrus homologs of R2R3-MYB73 and ATERF6 were constitutively up-regulated in the flavedo of AS lines and confirmed to be up-regulated by 3- and 7-fold, respectively, by qRT-PCR analyses (Fig. 5B; Supplemental Fig. S7). Genes encoding putative WRKY6 and WRKY33 transcription factors were induced more than 4- and 2-fold, respectively, but this was only observed in line AS7 (Fig. 5B; Supplemental Table S2B). Moreover, several “no apical meristem” (NAC domain) genes were up-regulated by approximately 2-fold in both lines (Fig. 5B; Table I; Supplemental Table S2). Most of these TFs have been related to the elicitation of secondary metabolism and defense (Bedon et al., 2010; De Geyter et al., 2012).
Additionally, a large proportion of the remaining misregulated genes in either AS line or that were common to both of them could be linked to the phenylpropanoid biosynthetic pathway. Congruently, several FAD-binding domain-containing proteins were up-regulated by more than 2-fold in AS lines (Fig. 5C; Table I; Supplemental Table S2). Although homologs of CHS (for chalcone synthase) and PAL1 (for Phe ammonia-lyase1) genes were up-regulated by approximately 2-fold in both AS lines, several flavonol O-methyltransferase homologs were clearly down-regulated by up to 3-fold (Fig. 5C; Tables I and II; Supplemental Table S2). PAL1 was confirmed to be up-regulated by 4- to 7-fold in qRT-PCR analyses (Supplemental Fig. S7). This finding is consistent with the well-known role of some of the up-regulated TFs as positive and negative regulators of enzymes required for the biosynthesis of phenylpropanoids (Grotewold, 2005).
Other defense-related genes, such as LTP1 (for nonspecific lipid transfer protein1) and NBS-LRR (for nucleotide-binding site Leu-rich repeat), were highly induced in the AS lines (Fig. 5B; Table I; Supplemental Table S2). LTP1 was confirmed to be up-regulated by 4-fold in subsequent qRT-PCR analyses (Supplemental Fig. S7), suggesting its possible involvement in the induction of disease resistance responses in AS citrus fruits. Regarding cell wall organization and biogenesis, several homologs of cellulose synthase and other xyloglucan endotransglycosylase genes were found to be up-regulated in both AS3 and AS7 fruits (Supplemental Table S2). Genes for other enzymes putatively involved in starch biosynthesis or electron transport were also up-regulated (Table I; Supplemental Table S2). Overall, these results indicate that terpene down-regulation activates constitutive defense responses in the fruit flavedo.
Down-Regulation of d-Limonene and Related Terpenes Triggers the Accumulation of JA in AS and SA in EV Orange Peels upon Fungal Inoculation
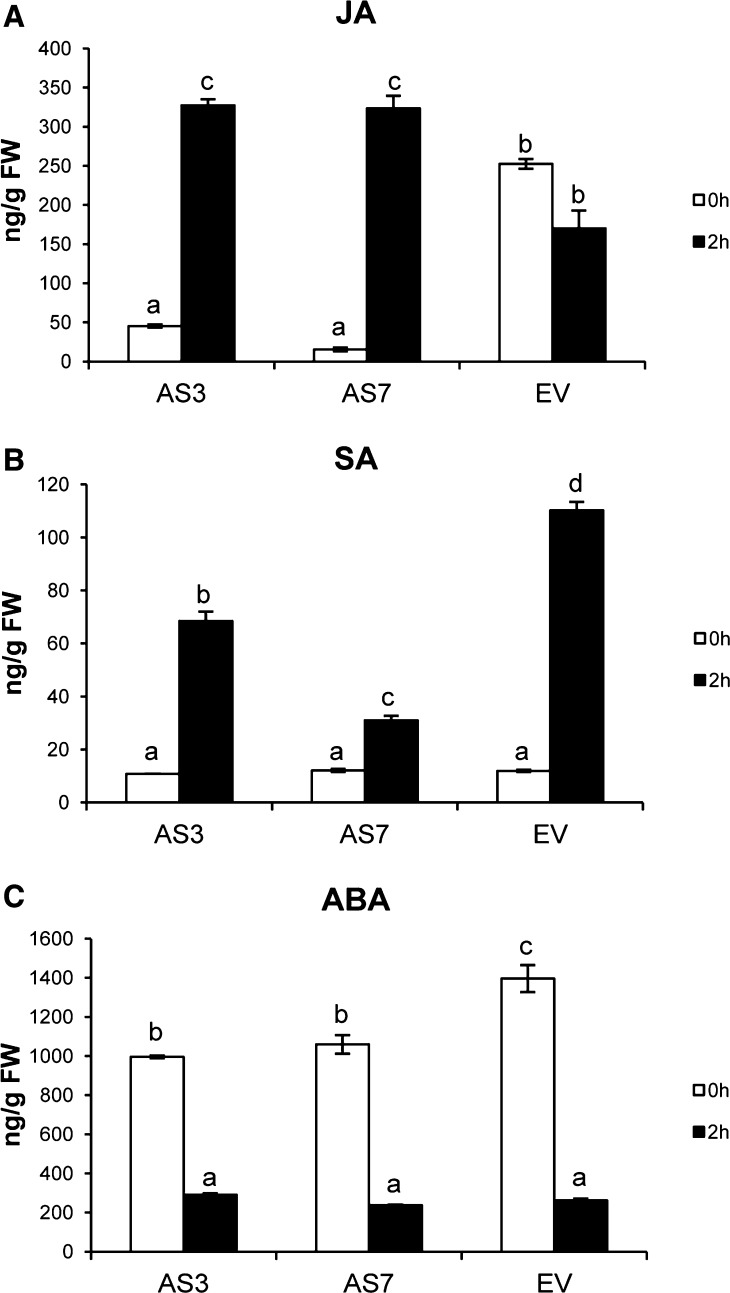
Because the up-regulation of specific TFs such as those described above has been linked to wound-, pathogen-, and herbivore-induced JA and SA accumulation, JA and SA together with abscisic acid (ABA) levels were quantified in the flavedo of AS and EV lines before and after inoculation with P. digitatum to assess whether these defense signaling molecules were activated or repressed by d-limonene down-regulation. Whereas low levels of JA were observed in AS fruits before fungal inoculation (compared with EV controls), an approximately 7- to 20-fold increase in JA content was observed in AS flavedo 2 h after wounding (from 45 to 327 ng g−1 fresh weight in the AS3 line and from 15 to 323 ng g−1 fresh weight in the AS7 line; Fig. 6A), reaching levels higher than those of flavedo of EV controls. A small decrease in JA levels was observed in the EV control samples after wounding (from 252 to 170 ng g−1 fresh weight), indicating that fungal infection had a minimal effect on JA levels in these fruits (Fig. 6A). Moreover, the analyses of JA accumulation in samples taken at 1 and 5 d after fungal challenge inoculation showed that the accumulation of JA in AS and EV control samples was comparable to that found at 2 h after wounding (Supplemental Fig. S8). These results were consistent in different independent transgenic lines over several months of two consecutive fruiting seasons (data not shown). The SA content was constitutively low in all samples but increased in the flavedo of both AS and EV lines 2 h after inoculation; however, SA increased much higher in EV samples (9-fold) compared with AS samples (6-fold in AS3; Fig. 6B). The attenuated increases of SA in AS samples observed upon inoculation may be related to the inhibitory effect of JA, as antagonistic interactions between these two compounds are common and well documented in plants (Glazebrook, 2005). ABA levels were slightly reduced in AS samples when compared with EV controls, although they were strongly decreased in all samples after inoculation (Fig. 6C). These results might be better explained by the cross talk of ABA with JA and/or SA signaling pathways and may not be directly related to constitutive monoterpene down-regulation (Anderson et al., 2004; Flors et al., 2008).
Figure 6.
Phytohormone measurement in the flavedo of AS and EV control fruits. JA (A), SA (B), and ABA (C) contents were measured before (0 h) and after (2 h) P. digitatum inoculation. Data represent means ± se and are derived from at least six fruits per plant. Different letters indicate significant differences at P < 0.05 using Fisher’s protected lsd test. FW, Fresh weight.
The AS Fruit Response to Fungal Challenge Inoculation Mimics the Exogenous Application of JA to Regular Fruit, Resulting in Protection against P. digitatum and the Up-Regulation of Genes of the JA Biosynthesis and Signaling Pathways
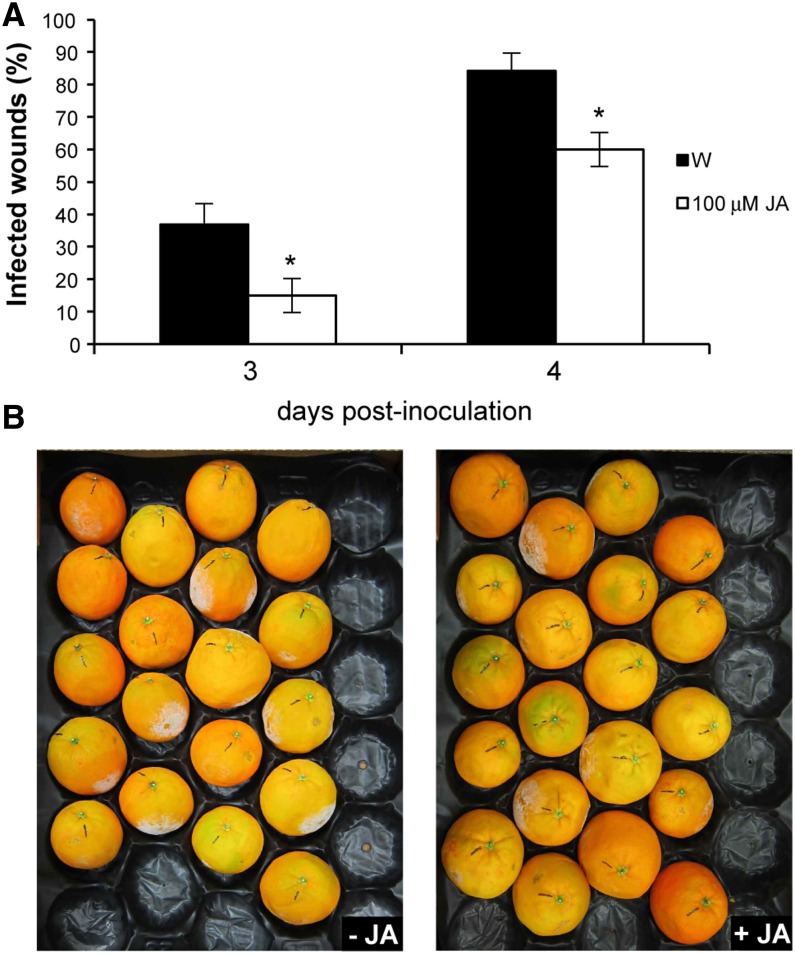
Pretreatment of plants with exogenous jasmonates such as methyl jasmonate or JA has been shown to induce protection against different necrotroph pathogens in many plants, including Arabidopsis and grapefruit (Citrus paradisi; Thomma et al., 1998; Droby et al., 1999). To examine the effect of the exogenous application of JA on induced resistance in citrus fruits, regular untransformed cv Navelina oranges and Clementine mandarins (Citrus clementina) were wounded, either water or JA was immediately applied to the wounds, and then fruits were inoculated with P. digitatum. Pretreatment with JA conferred significant levels of induced resistance, as shown by the reduced percentage of infection in pretreated fruits 3 and 4 d after challenge inoculation (Fig. 7; Supplemental Fig. S9).
Figure 7.
Exogenous application of JA in regular orange fruits confers fungal resistance. The evolution of the disease caused by the fungus P. digitatum in mature orange fruits inoculated with 1 × 105 spores mL−1 and pretreated with water (W) or JA is shown. A, Percentage of infected wounds in inoculated points 3 and 4 d after inoculation. The results are averages ± se (n ≥ 19). *P < 0.05 using Student’s t test. We repeated all experiments at least twice and obtained similar results. B, Regular fruits 4 d after inoculation.
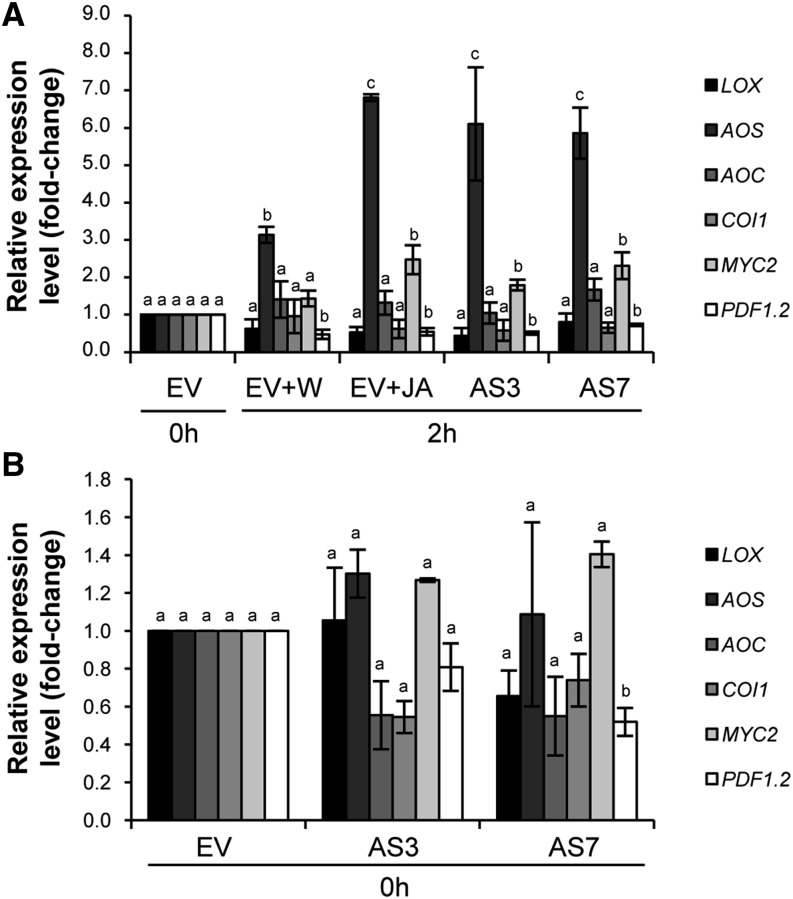
To verify that the observed resistance was directly related to the application of JA, the expression levels of several genes of the JA biosynthesis and signaling pathways were analyzed by qRT-PCR in AS and EV fruits before and after fungal inoculation. Transcript levels of AOS, coding for the bottleneck enzyme of the pathway (Schaller, 2001), were rapidly induced in both “inoculated + water” and “inoculated + JA” EV samples 2 h after treatment, but levels of AOS transcripts were much higher in JA- than in water-treated fruits (6.8- versus 3.1-fold change, respectively; Fig. 8A), indicating that exogenous application of JA positively boosted JA biosynthesis. Among TFs acting downstream of JA in the stress responses, the bHLHzip-type transcription factor AtMYC2, a master switch in JA signaling, is essential in regulating networks that modulate phytohormone and secondary metabolism in plants (De Geyter et al., 2012; Wasternack and Hause, 2013). Additionally, AtMYC2 negatively regulates the plant defensin gene (PDF)1.2 (Lorenzo et al., 2004). The sweet orange homolog of MYC2 was up-regulated in JA-treated EV samples, whereas PDF1.2 was down-regulated in both water- and JA-treated EV fruits. No differences were found in the relative expression of the other genes analyzed (Fig. 8A).
Figure 8.
Quantitative real-time PCR analyses of genes involved in JA biosynthesis (sweet orange homologs of LOX, AOS, and AOC) and signaling (sweet orange homologs of COI1, MYC2, and PDF1.2). The differential expression of JA biosynthetic/signaling genes in EV control and AS fruits after (A) and before (B) P. digitatum inoculation is shown. EV fruits were treated either with water (EV+W) or JA (EV+JA) before challenge inoculation. The expression of each gene was analyzed using four different 96-well plates (with at least three technical replicates for each one). Data represent means ± se and are derived from at least three fruits per plant. Fold change was calculated in relation to two independent EV control lines before inoculation, to which an arbitrary value of 1 was assigned. Different letters indicate significant differences in the expression of each gene at P < 0.05 using Fisher’s protected lsd test. AS3 and AS7 refer to two independent d-limonene synthase AS transgenic lines.
After inoculation with P. digitatum, AS samples showed a marked AOS activation (6.1- and 5.9-fold change in AS3 and AS7, respectively), an increase in MYC2 expression (1.8- and 2.3-fold change), and a slight decrease in PDF1.2 expression, similar values to those found in JA-treated EV fruits (Fig. 8A), even when JA concentrations applied to them were much higher than the physiological values.
Additionally, whether JA biosynthesis and signaling genes were misregulated in the transgenic AS versus EV control fruits before inoculation with P. digitatum was also investigated. Most genes of the JA pathway showed only slight, nonsignificant differences in expression, which was consistent with the microarray data. Significant down-regulation was found only in the case of the PDF1.2 gene in AS7 (Fig. 8B). One explanation for the discrepancy in basal JA accumulation (but not JA pathway gene expression) between AS and EV fruits is perhaps related to the posttranscriptional or posttranslational modification of certain enzymes in the JA biosynthesis pathway, affecting enzyme abundance and/or activity (Yang et al., 2012).
d-Limonene Supplementation to Either AS or EV Fruit Strongly Increases JA Levels
To evaluate whether low basal JA accumulation and its dramatic increase upon fungal challenge was a direct consequence of the constitutive d-limonene down-regulation in AS oranges, we applied pure (R)-(+)-limonene to either AS or EV orange peels and measured JA levels at 20 min and 2 and 4 h post injection. (R)-(+)-Limonene supplementation induced JA accumulation not only in AS but also in EV samples (Fig. 9A). Although the method used to inject (R)-(+)-limonene in fruits caused some stress to the peels (Fig. 9B), which might explain in part the increased JA levels observed at 20 min, further enhanced JA levels in all samples at 2 h post injection suggested a possible direct link between d-limonene levels and the activation of JA metabolism. Figure 9A also shows that the effects of (R)-(+)-limonene application on increased accumulation of JA levels were transient, because JA levels decreased significantly in AS3 and AS7 fruit peels (but not in EV ones) at 4 h post injection, suggesting a tendency of AS fruits to recover their constitutive low JA levels (Fig. 6A).
Figure 9.
Exogenous application of (R)-(+)-limonene in the EV control and AS transgenic orange fruits promotes the accumulation of JA. A, Accumulation of JA in mature orange fruits treated with water (W) or (R)-(+)-limonene (L) at different hours after the application (20 min and 2 and 4 h). The results are averages ± se (n ≥ 15). Different letters indicate significant differences at P < 0.05 using Fisher’s protected lsd test. FW, Fresh weight. B, Control fruits 2 h after water or (R)-(+)-limonene application. The dashed circle indicates the stress caused by the application of the pure compound.
d-Limonene Down-Regulation Activates Both Constitutive and Fungus-Inducible Geranylgeranyl Diphosphate Synthase Up-Regulation
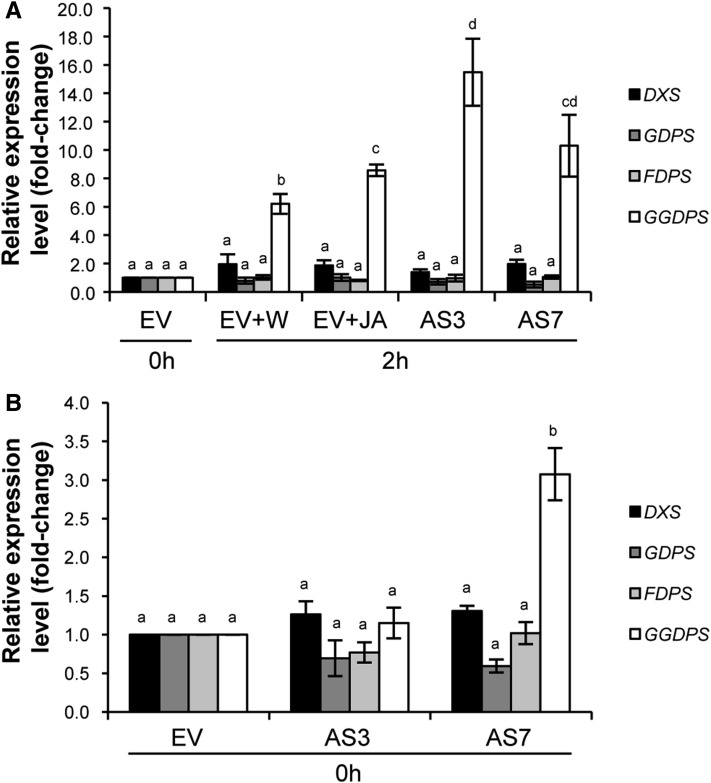
To assess whether d-limonene down-regulation altered isoprenoid pathways in both JA-treated EV and nontreated AS fruits upon fungal challenge inoculation, the expression of sweet orange homologs of 1-deoxyxylulose 5-phosphate synthase (DXS), geranyl diphosphate synthase (GDPS), farnesyl diphosphate synthase (FDPS), and geranylgeranyl diphosphate synthase (GGDPS) was analyzed by qRT-PCR in inoculated + water and inoculated + JA EV fruits versus inoculated AS fruits and in unchallenged EV versus AS fruits. Inoculation up-regulated GGDPS in control fruits, but JA application enhanced this effect significantly (Fig. 10A). However, it was 2 h after inoculation when GGDPS expression was dramatically increased, by more than 10-fold, in both AS lines compared with EV nontreated controls (Fig. 10A).
Figure 10.
Quantitative real-time PCR analyses of genes involved in isoprenoid biosynthesis (sweet orange homologs of DXS, GDPS, FDPS, and GGDPS). The differential expression of isoprenoid genes in EV control and AS fruits after (A) and before (B) P. digitatum inoculation is shown. EV fruits were treated either with water (EV+W) or JA (EV+JA) before challenge inoculation. The expression of each gene was analyzed using four different 96-well plates (with at least three technical replicates for each one). Data represent means ± se and are derived from at least three fruits per plant. Fold change was calculated in relation to two independent EV control lines before inoculation, to which an arbitrary value of 1 was assigned. Different letters indicate significant differences in the expression of each gene at P < 0.05 using Fisher’s protected lsd test. AS3 and AS7 refer to two independent d-limonene synthase AS transgenic lines.
GGDPS was constitutively up-regulated in AS7 but not in AS3 compared with EV control samples (Fig. 10B). No significant differences were found in the expression of the other prenyl transferase genes in JA-treated and transgenic AS fruits either exposed or not exposed to fungal challenge when compared with corresponding controls (Fig. 10).
These results led us to further explore the constitutive GGDPS up-regulation in AS orange peels. As the fruit developmental stage in which d-limonene reaches its peak in regular fruit is green shortly before the breaker stage (Attaway et al., 1967; Kekelidze et al., 1989; Rodríguez et al., 2011b) and green flavedo showed fungal resistance comparable to that exhibited by mature fruit in AS transformants (Fig. 1), GGDPS levels were analyzed by qRT-PCR in AS versus EV green fruit. Significant increases were found in GGDPS levels in both AS3 (4.4-fold) and AS7 (19.9-fold) peels compared with those in EV samples. Therefore, decreased d-limonene accumulation in AS fruits triggered alterations in the expression of GGDPS and likely in GGDP-derived isoprenoid metabolism.
DISCUSSION
Terpenoids are ecologically important molecules (Degenhardt et al., 2003) due to various properties such as their volatility, flavor/aroma, and toxicity, which give them important roles in plant defense, plant-to-plant communication, and pollinator attraction (Pichersky and Gershenzon, 2002). Transgenic plants with modified terpenoid production can contribute to fundamental studies aimed to understand their function in plant/environment relationships (Aharoni et al., 2005). In a previous study, we showed that the transgenic down-regulation of a d-limonene synthase gene led to a dramatic reduction in the levels of d-limonene and other monoterpene and sesquiterpene hydrocarbons, whereas monoterpene alcohols, such as nerol or citronellol, were substantially increased. Consequently, fruits were more resistant to P. digitatum and bacterial pathogens (Rodríguez et al., 2011a, 2011b). Resistance to P. digitatum was related to d-limonene down-regulation and not to the increased accumulation of monoterpene alcohols, as in vitro assays with pure (R)-(+)-limonene, nerol, or citronellol showed that these compounds were not toxic to the fungus but, instead, had a pronounced stimulatory effect on the germination of P. digitatum spores that was directly related to the concentration of the pure compound used.
Here, we have found that d-limonene down-regulation increased GGDPS levels in green orange peels without altering oil gland morphology and the concentration of carotenoids or the phytol side chains of chlorophylls. This suggests that the likely accumulation of GGDP in AS oranges could provide substrates for the biosynthesis of diterpenoids but not for phytoene or phytol. Diterpenes are widely recognized as antimicrobial compounds in forest trees (Trapp and Croteau, 2001). Silencing of GGDPS in Nicotiana attenuata allowed hornworn (Manduca sexta) larvae fed on their leaves to gain up to three times more mass than those that fed on control plants, through suppression of the synthesis of diterpenoids derived from GGDP function (Jassbi et al., 2008). Likewise, constitutive up-regulation of GGDPS in orange fruits may supply a substrate for the accumulation of antimicrobial diterpenes in oil glands that would act as either direct or indirect defenses against P. digitatum and other fungi. In this study, the drop in d-limonene accumulation in mature AS fruits produced enhanced up-regulation of GGDPS after challenge inoculation with P. digitatum. Because AS fruits exhibited a broad resistance to different pathogens and pest repellency (Rodríguez et al., 2011b), it would be interesting to test whether GGDPS could also be further activated by such different pathogens and/or under distinct plant-pathogen-pest interaction scenarios in AS peels. Moreover, identifying such putatively GGDP-derived diterpenes would be of major biotechnological importance. It has been recently demonstrated that abietane diperpenoids, which are major constituents of oleresin from conifers, are activators of immunity against pathogens in Arabidopsis, tomato (Solanum lycopersicum), and tobacco (Nicotiana tabacum; Chaturvedi et al., 2012). The down-regulation of a d-limonene synthase gene in orange flavedo also triggers innate immunity defense cascades likely linked to the accumulation of GGDP-derived diterpenoids. The molecular basis for this cross talk between isoprenoid metabolism and the signaling pathways that mediate pathogen resistance is unknown.
Microarray-mediated transcriptional profiling has been successful in identifying constitutively activated defense signaling pathways in the flavedo of AS citrus fruit potentially related to the increased fungal resistance. Characteristic CDPK and MAPK cascades were up-regulated in AS samples in addition to early response and protein phosphatase kinase targets (Asai et al., 2002; Boudsocq et al., 2010) that phosphorylate TFs belonging to the R2R3-MYB, MYC, WRKY, ERF/AP2, and NAC domains and the zinc finger (CCCH-type) families, which likely participate in defense (Wang et al., 2008; Guo et al., 2009; Birkenbihl and Somssich, 2011; De Geyter et al., 2012). Many of these TFs, often JA activated themselves, have been related to the JA-modulated regulation of defense and/or elicitation of secondary metabolism. For example, the homolog MYB14 (as well as other members of the R2R3-MYB TF family with a conserved EAR motif) is activated in the conifer trees white spruce (Picea glauca) and loblolly pine (Pinus taeda) after JA application (or wounding when overexpressed transgenically) and contributes to the accumulation of terpenoids and broad defense responses (Bedon et al., 2010). WRKY33, a JA- or pathogen-inducible TF, is required for the MPK3/MPK6 induction of biosynthesis of the major defense compound camalexin in Arabidopsis (Mao et al., 2011), and the silencing of WRKY3 or WRKY6 generates plants highly vulnerable to herbivores by impairing JA accumulation and the synthesis of sesquiterpene volatiles in N. attenuata (Skibbe et al., 2008).
Although the accumulation of JA and transcripts encoding the sweet orange homolog of AOS and MYC2 dramatically increased in AS fruits after inoculation with P. digitatum, resembling the response of EV fruits to JA treatment, and application of JA conferred fungal resistance to untransformed orange fruits, we were unable to establish a direct relation between d-limonene down-regulation, constitutive reduction in JA metabolism, and fungal resistance in AS fruits, because pure (R)-(+)-limonene application to orange peels drastically increased instead of decreased JA levels. It could be argued that this treatment may have caused too much stress to orange peels, which could have interfered with the results of the experiment. Impairing JA accumulation/signaling by producing transgenic orange plants with AOS- or MYC2-deficient fruits and testing d-limonene accumulation in their peel oil glands would better clarify the cross talk between JA, d-limonene, and fungal resistance in orange peels. Regarding the activation of JA metabolism in AS fruits (while SA is activated in EV fruits) upon challenge inoculation, it can only be explained when our understanding of the pathways involved and their possible interactions are more complete. It may be possible that JA signaling has dual functions in orange fruit-P. digitatum interactions, participating in susceptibility if activated early or constitutively (as in EV fruits) and in defense during later stages of infection, as has been clearly demonstrated for the interaction of either wheat (Triticum aestivum) or Arabidopsis and the fungal pathogen Fusarium graminearum (Makandar et al., 2012). Transcriptomic and metabolomic analyses of AS versus EV fruit peels at different time points after fungal challenge inoculation may help in finding connections among isoprenoid metabolism, defense-related pathways, and phytohormone signaling cascades.
Another mechanism by which d-limonene down-regulation may activate defenses against pathogens is through the up-regulation of the disease resistance/LRR protein gene family (Chen et al., 2002) or other genes that code for proteins that are either antimicrobial themselves or that catalyze the production of antimicrobial compounds such as LTP1, which was up-regulated in AS lines and is predicted to be a member of the PR14 pathogenesis-related protein family. Various LTPs have been shown to have in vitro antimicrobial activity against fungi and bacteria (Sels et al., 2008). LTP1 is localized in the cell wall and binds calmodulin in a Ca2+-independent manner (Thoma et al., 1994; Wang et al., 2004). The endogenous overexpression of three LTP-like genes in Arabidopsis resulted in enhanced tolerance to Botrytis cinerea (Chassot et al., 2007). Moreover, transgenic Arabidopsis plants overexpressing a barley (Hordeum vulgare) LTP1 gene exhibited enhanced resistance against Pseudomonas syringae pv tomato and B. cinerea (Jung et al., 2005).
To understand the basis of the induction of resistance against P. digitatum in citrus fruits, Ballester et al. (2011) using a 12K citrus cDNA microarray studied transcriptional changes in elicited fruits. Elicitation consisted of inoculation with the fungus followed by a curing treatment 1 d later (37°C for 3 days with high relative humidity) that strongly reduced the incidence of green mold in oranges. Several days after infection, the most highly induced genes belonged to the phenylpropanoid and ethylene pathways. Although wounding, infection, and successive curing treatments would likely cause the up-regulation of many stress-responsive genes, including those from both pathways, the expression of PAL1, the first gene in the phenylpropanoid pathway, was consistently increased in elicited fruits along with other downstream genes. We show here that some genes of the phenylpropanoid pathway, such as PAL1, together with CHS, were constitutively activated in the flavedo of d-limonene AS fruits before inoculation with P. digitatum. Other putative genes of the pathway, such as Cinnamate 4-Hydroxylase, Cinnamoyl CoA Reductase, and Reduced Epidermal Fluorescence8, were also slightly (approximately 1.5-fold) up-regulated in our transcriptional profiling analyses, although several O-methyltransferases were down-regulated in the case of AS fruits. Elicited and AS fruits showed altered phenylpropanoid biosynthetic pathways, indicating a correlation between the response to curing treatment and the constitutive defense activated by d-limonene down-regulation, which led in both cases to resistance against P. digitatum.
Transgenic plants with constitutive changes in terpenoid production often present altered phenotypes compared with their corresponding wild-type counterparts (Estévez et al., 2001; Aharoni et al., 2003; Wu et al., 2006). One possible explanation for the growth retardation phenotype in these transgenic lines is the depletion of the precursors, which may lead to reductions in the levels of essential compounds. These compounds include growth regulators and other vital components such as carotenoids, chlorophyll, and quinones. However, transgenic orange fruits with strong d-limonene synthase down-regulation did not exhibit growth retardation or changes in chlorophyll/carotenoid levels when compared with control fruits. Congruently, prenyl transferase genes other than GGDPS were not consistently misregulated in AS fruits before and after fungal challenge inoculation. These results indicate that in orange fruits, neither a reduction in d-limonene nor its metabolic consequences caused morphological alterations or other pleiotropic effects. In the same sense, the constitutive up-regulation of TFs, such as MYBs or WRKYs, was sufficiently moderated in AS oranges to avoid the diversion of resources away from fruit growth and development. Such phenotypical alteration conversely occurred in transgenic plants either up-regulating or down-regulating genes encoding similar TFs (Bedon et al., 2010; Kallenbach et al., 2010; Yang et al., 2012).
In nature, the d-limonene concentration is lower in the first stages of orange development. However, once the fruit has almost attained its final size and the seeds are fully viable, d-limonene levels drastically increase, and it becomes the predominant constituent of flavedo oil glands until fruit maturation (Dugo and Di Giacomo, 2002; Flamini and Cioni, 2010; Rodríguez et al., 2011b). We have shown here that high d-limonene and related terpene levels are tightly associated with a general depletion of defense-related genes. Because an increase in d-limonene occurs once the seed is formed and this coincides with a general enhanced susceptibility to opportunistic pathogens (Rodríguez et al., 2011b), our results indicate that the high accumulation of d-limonene and related terpenes might be a signal that attracts vertebrate frugivores and facilitates infection by specialized microorganisms. As the accumulation of d-limonene at high levels in flavedo of mature fruits is common to all Citrus species, including relatives and ancestral types (Dugo and Di Giacomo, 2002), our results may also indicate that P. digitatum and other microorganisms have acted to shape the evolution of d-limonene content in citrus fruit peel. Whether this additionally serves to attract legitimate vertebrate dispersers or facilitates their access to the fruit pulp and seeds requires further investigation.
MATERIALS AND METHODS
Plant Material
Fruits of independent d-limonene synthase AS and EV control lines of sweet orange (Citrus sinensis cv Navelina and Pineapple) at different developmental stages (August to December; Supplemental Fig. S1) were harvested over 3 consecutive years. To determine the oil gland size and number in orange peels, a defined area of 200 mm2 along the equator of the fruit was measured using 10 fruits for each developmental stage. Gland density was measured using fruits of 70 and 90 mm in diameter (green and mature cv Navelina flavedo, respectively) and 60 and 80 mm in diameter (green and mature cv Pineapple flavedo, respectively). Images were taken with a Leica DFC490 digital camera mounted on a magnifying glass, and secretory glands visible on the surface were counted and measured using the UTHSCSA ImageTool software (version 3.0; Department of Dental Diagnostic Science at the University of Texas Health Science Center). Gland density was expressed as number of glands per cm2. Peel thickness was measured with a caliper (as both flavedo and albedo or flavedo only) in four different sections around the equator of 10 mature fruits.
For the analysis of chlorophyll and total carotenoid content, the flavedo tissue (outer colored part of the fruit peel) was separated from the fruits. The flavedo was frozen in liquid nitrogen, ground to a fine powder, and stored at −80°C until analysis. The data for chlorophyll and carotenoid content are presented as means ± se of 10 replicate samples.
Fruit color was measured using a Minolta CR-200 chromameter on three locations around the equatorial plane of the fruit. Hunter laboratory parameters L (0–100; black to white), a (±; yellow/blue), and b (±; red/green) were determined, and color index was expressed as the results of the 1,000 × a/Lb transformation. Negative values represent green color, zero is the breaker point, and positive values indicate yellow to orange coloration. Fruit color was registered using 20 replicate samples for each developmental stage.
For phytohormone quantification in fruit flavedo, mature fruits that were 90 mm in diameter were used. Flavedo samples were excised with a razor blade before (0 h) and after (2 h) inoculating the fruit in the equatorial region with a stainless steel rod as described (Rodríguez et al., 2011b). Data were obtained from the analysis of at least six fruits per line, and this analysis was repeated several times during the fruiting season and over 2 consecutive years.
Fungal Assays
For the experiments of natural infection by fungi, fruits were harvested monthly during a 5-month period for 2 consecutive years. Ten fruits per independent transgenic line were used for each experiment. Fruits were partially peeled and put into plastic trays for germination of ubiquitous spores. Observations were made daily for the appearance and progress of symptoms. Samples placed on slides for microscopic identification were obtained from fungi-infected fruits.
For the assays of JA supplementation to the regular cv Navelina sweet orange control and Clementine mandarin (Citrus clementina) fruits, 25 μL of a 100 μm JA (Sigma-Aldrich) aqueous solution containing 0.1% Tween 20 (Sigma-Aldrich) as surfactant was allowed to penetrate in the wound, and the same procedure for inoculation and incubation described before (Rodríguez et al., 2011b) was followed. Inoculation was performed with 1 × 105 spores mL−1. For control treatment, a solution of water supplemented with Tween 20 (0.1%, v/v) was used. For each treatment, replicates of at least 18 and 30 fruits per line were used with orange and Clementine mandarin, respectively. Disease incidence was estimated as the number of infected wounds per total number of inoculated points.
Chlorophyll and Total Carotenoid Extraction and Quantification
Fruit pigments were extracted as described previously (Rodrigo et al., 2003). Briefly, the chlorophyll (a + b) content was determined by measuring the absorbance at 644 and 662 nm and calculated according to the equations of Smith and Benitez (1955). After chlorophyll measurements, the pigment ethereal solution was dried and saponified using a 10% methanolic KOH solution. The carotenoids were subsequently reextracted with diethyl ether until the hypophase was colorless. An aliquot of the ethereal extract was used for the quantification of total carotenoid content by measuring the absorbance of the saponified extracts at 450 nm using the extinction coefficient of β-carotene, E1% = 2,500 (Davies, 1976). The samples were dried under N2 and kept at −20°C until HPLC analysis. All operations were carried out on ice under dim light to prevent photodegradation, isomerization, and structural changes in the carotenoids.
HPLC of Carotenoids
For HPLC analysis of carotenoids, the peels of fruits at two maturation stages were selected as follows: fruits harvested in August (green) and fruits harvested in December (mature). The samples were prepared for HPLC by dissolving the dried residues in methanol:acetone (2:1, v/v). Chromatography was carried out using a Waters liquid chromatography system equipped with a 600E pump, a model 996 photodiode array detector, and Millennium Chromatography Manager software (version 2.0; Waters) as described previously (Rodrigo et al., 2004). A C30 carotenoid column (250 mm × 4.6 mm, 5 μm) coupled to a C30 guard column (20 mm × 4.0 mm, 5 μm; YMC Europe) were used with methanol, water, and methyl tert-butyl ether. Carotenoid pigments were analyzed by HPLC using a ternary gradient elution that was reported previously (Rouseff et al., 1996). The photodiode array detector was set to scan from 250 to 540 nm throughout the entire elution profile. The area of each peak was obtained, and the percentage of each individual carotenoid was calculated over the total area of carotenoid peaks, as integrated by the Maxplot chromatogram. Each sample was extracted twice, and two replicate injections from each extraction were performed. The carotenoid peaks were integrated at their individual maxima wavelength, and their content was calculated using calibration curves of zeaxanthin (Sigma) for zeaxanthin, β-carotene (Sigma) for α-carotene and β-carotene, β-cryptoxanthin (Extrasynthese) for α-cryptoxanthin and β-cryptoxanthin, and lutein (Sigma) for lutein, violaxanthin, and neoxanthin isomers. Phytoene and phytofluene were purified previously, as described by Pascual et al. (1993), by thin-layer chromatography from carotenoid extracts of pinalate orange fruit, a mutant that accumulates substantial amounts of these carotenes (Rodrigo et al., 2003).
Extraction of Volatiles and Gas Chromatography-Mass Spectrometry Analysis
Flavedo tissue was obtained from orange fruits, immediately frozen in liquid nitrogen, and stored at −80°C until extraction. A Thermo Trace GC Ultra coupled to a Thermo DSQ mass spectrometer with the electron ionization mode set at 70 eV was used. Extraction and analysis were carried out as described before (Rodríguez et al., 2011b). Briefly, frozen ground material (200 mg) was weighed in screw-cap Pyrex tubes, and then 3 mL of cold pentane and 25 μg of 2-octanol (Fluka) were immediately added as an internal standard. Samples were homogenized on ice for 30 s with a Yellowline homogenizer (model DI 25). The suspension was vortexed for 15 s, and 3 mL of MilliQ water was added. The sample was further vortexed for 30 s and centrifuged at 1,800g for 10 min at 4°C. The organic phase was recovered with a Pasteur pipette, and the aqueous phase was reextracted two more times with 3 mL of pentane. A 2-μL aliquot of the pooled organic phases was directly injected into the gas chromatograph-mass spectrometer for volatile analysis; at least two extractions for each sample were performed.
The ion source and the transfer line were set to 200°C and 260°C, respectively. Volatile compounds were separated on an HP-INNOWax (Agilent J&C Columns) column (30 m × 0.25 mm i.d. × 0.25 μm film). The column temperatures were programmed as follows: 40°C for 5 min, raised to 150°C at 5°C min−1, then raised to 250°C at 20°C min−1, and held for 2 min at 250°C. The injector temperature was 220°C. Helium was the carrier gas at 1.5 mL min−1 in the splitless mode. Electron-impact mass spectra were recorded in the 30- to 400-atomic mass unit range with a scanning speed of 0.5 scan−1. Compounds were identified by matching the acquired mass spectra with those stored in the reference libraries (Wiley6 and the National Institute of Standards and Technology) or from authentic standard compounds when available. Data were quantified by integrating the peak areas of total ion chromatograms and normalizing to the recovery rate of the internal standard (2-octanol). The data in Figures 1 and 2, Supplemental Table S1, and Supplemental Figure S2 represent the relative amounts of individual terpenes and are presented as the percentage area of each terpene (given as a fraction of unity) with respect to the total terpene peak area for monoterpene hydrocarbons in the EV line, which was assigned an arbitrary value of 1.
RNA Extraction
Total RNA was isolated from flavedo as described previously (Rodrigo et al., 2004). For real-time qRT-PCR analyses, RNA was cleaned up with the RNeasy mini kit (Qiagen) and treated with DNase I (Rnase-Free DNase Set; Qiagen) following the manufacturer’s instructions. RNA was quantified using a Nanodrop spectrophotometer (NanoDrop 2000C; ThermoScientific).
Microarray Experimental Design, Hybridization, Data Acquisition, and Data Analysis
Microarray experiments were performed with the mature orange flavedo (90 mm in diameter) of two independent AS (AS3 and AS7) and EV (EV1 and EV2) transgenic lines, comparing AS versus EV samples on the same slide. A pool of flavedo from fruits of three plants per line was used in every experiment. Three slides for every comparison (AS3 versus EV1 and AS7 versus EV2) were used. The total RNA from each line was duplicated for dye-swap labeling.
Gene expression analysis was conducted using a citrus cDNA microarray containing 21,081 putative unigenes (Martinez-Godoy et al., 2008). Microarray labeling, hybridization, and scanning were performed as described previously (Forment et al., 2005). Microarray slides were scanned with a GenePix 4000B scanner (Molecular Devices) using GenePix 6.0 image-acquisition software. Spots with a net intensity in both channels that was lower than the median spot signal background plus 2 sd were not used for further analysis. Data were normalized using an intensity-based Lowess function, a normalization procedure based on robust local regression, to accommodate different types of dye biases and the use of control sequences spotted on the array (Yang et al., 2002) and analyzed only for features with at least three values. Differentially expressed genes were identified using the one-class significance analysis of microarrays test (Tusher et al., 2001). A common set of genes was identified based on the overlap between the lists from each transgenic line. A gene was considered to be differentially expressed if the false discovery rate was less than 5% and it had at least a 1.6-fold average change in expression between AS and EV plants.
Functional Categorization of Differentially Expressed Genes
Genes that were differentially expressed were grouped into GO categories according to their biological function. Because very limited functional information is available for the sequences represented on the citrus genome array, the transcripts were annotated by finding orthologs in Arabidopsis (Arabidopsis thaliana) using The Arabidopsis Information Resource.
Results from Fatiscan analyses (Medina et al., 2010) were used to represent statistically significant GO biological processes from levels 3 to 9. GO categories were grouped into five main groups (Tables I and II): “defense response,” which covers GO categories such as defense response, response to biotic stimulus, immune response, plant-type hypersensitive response and death, response to abiotic stimulus, response to stress, response to chemical stimulus, response to endogenous stimulus, and response to external stimulus; “cellular component organization and biogenesis,” which covers GO categories such as establishment of localization, cellular component organization and biogenesis, plant-type cell wall organization, and cell communication; “other,” which covers GO categories such as cellular metabolic process, primary metabolic process, regulation of biological process, regulation of transcription, macromolecule metabolic process, regulation of biological quality, and nitrogen compound metabolic process; “secondary metabolic process,” which covers GO categories such as monoterpenoid biosynthetic process, sesquiterpenoid biosynthetic process, and phenylpropanoid biosynthetic process; and “unknown,” which covers genes without a match in the databases.
Real-Time qRT-PCR
The expression of selected genes chosen from microarray analyses was estimated by real-time qRT-PCR using the SYBR Green assay and the LightCycler480 System (Roche) equipped with LightCycler 480 version 1.5 software. The genes selected were LIMONENE SYNTHASE, GERMACRENE-d SYNTHASE, CALMODULIN, MYB73 transcription factor, PAL1, LTP1, and ATERF6. For JA signaling and biosynthesis and for isoprenoid biosynthesis, the homolog genes from citrus, LOX, AOS, AOC, COI1, MYC2, a bHLHzip-type MYC transcription factor, PDF1.2, DXS, GDPS, FDPS, and GGDPS, were selected. The primers were designed based on the corresponding sequences available in the database of the Citrus Functional Genomics Project (http://bioinfo.ibmcp.upv.es/genomics/cfgpDB; Supplemental Table S3).
For the microarray genes selected, one-step reverse transcription-PCR was carried out with 25 ng of DNase-treated RNA by adding 1.6 units of SuperScript II Reverse Transcriptase (Invitrogen), 0.8 units of Protector RNase Inhibitor (Roche), 6.25 μL of Power SYBR Green PCR Master Mix (Applied Biosystems), and optimized amounts of gene-specific primers (Supplemental Table S3) in a total volume of 12.5 μL. Incubations were carried out as follows: 45°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 40 s, and 70°C for 15 s. Fluorescence intensities were acquired during the 70°C step.
For the JA and terpenoid biosynthetic genes selected, two-step reverse transcription-PCR was carried out. First-strand cDNA was synthesized from 1 μg of total RNA using SuperScript II Reverse Transcriptase following the manufacturer’s protocol. cDNA samples were diluted 1:5 with nuclease-free water before analysis, and 2 μL of this dilution was used for the subsequent steps. Reactions were carried out with 10 μL of LightCycler 480 DNA SYBR Green I Master (Roche) and 2 μL of gene-specific primers (Supplemental Table S3) in a total volume of 20 μL. Incubations were carried out as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 20 s. Fluorescence intensities were acquired during the 72°C step.
The specificity of the amplification reactions was assessed by postamplification dissociation curves. To transform the fluorescence intensity measurements into relative mRNA levels, a standard curve was generated with a 10-fold dilution series of an RNA sample. Relative mRNA levels were normalized to the citrus actin gene (GenBank accession no. CX289161) following the efficiency method (Pfaffl, 2001).
Induction values of 1-fold were arbitrarily assigned to the control sample before inoculation. The quantification of each transcript in each cDNA source was accomplished using at least three independent biological replicates and nine technical replicates for each one with two AS and two EV control independent lines. Means ± se values were calculated.
Phytohormone Quantification
Hormone extraction and analysis were carried out as described by Durgbanshi et al. (2005), with slight modifications. Briefly, 0.5 g of frozen plant material was extracted in 5 mL of distilled water after spiking with a mixture of 2H6-ABA, 2H6-SA, and dihydrojasmonic acid as internal standards. After centrifugation at 4,000g at 4°C, supernatants were recovered and the pH was adjusted to 3.0 with 30% acetic acid. The acidified water extract was partitioned twice with 3 mL of diethyl ether. The organic upper layer was recovered and evaporated under vacuum in a centrifuge concentrator (SpeedVac; Jouan). The dry residue was then resuspended in a 10% methanol solution by gentle sonication. The resulting solution was filtered through regenerated cellulose 0.22-μm membrane syringe filters (Albet) and directly injected into the HPLC system (Alliance 2695; Waters). Separations were carried out on a C18 column (Kromasil 100; 5 μm particle size, 100 × 2.1 mm; Scharlab) using a gradient of methanol-water supplemented with 0.01% acetic acid at a flow rate of 300 μL min−1. Hormones were quantified with a Quattro LC triple quadrupole mass spectrometer (Micromass) connected online to the output of the column through an orthogonal Z-spray electrospray ion source (Arbona et al., 2010).
For the assays of limonene supplementation, 5 μL of the pure compound [(R)-(+)-limonene; Sigma-Aldrich] or water was applied to a wound made in the equator of each EV control and AS transgenic fruit. For each treatment, replicates of 15 to 20 fruit per line were used. Inoculated fruits were placed on plastic cavity trays on open cardboard trays that prevent fruit contact and incubated at 20°C and 80% relative humidity for 4 h. Samples for phytohormone quantification were taken from all the lines at 20 min and 2 and 4 h after the application of (R)-(+)-limonene.
Statistical Analysis
Data on the characterization of orange peels, qRT-PCR, phytohormone levels, or arcsine-transformed data on the percentage of infected wounds in JA assays were subjected to ANOVA using Statgraphics version 5.1 software (Manugistics). Student’s t test or Fisher’s protected lsd test (P < 0.05) was used to separate the means, when appropriate.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number CX289161 (citrus actin).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Color changes during fruit development and maturation of sweet oranges (C. sinensis cv Navelina).
Supplemental Figure S2. Phenotype of green and mature flavedo in AS and EV Pineapple sweet orange fruits.
Supplemental Figure S3. Schematic representation of the metabolic diversion of the terpenoid pathway.
Supplemental Figure S4. Total chlorophyll and carotenoid content in cv Pineapple sweet orange fruits in green and mature flavedo.
Supplemental Figure S5. Percentage of carotenoids in cv Navelina and Pineapple sweet orange AS and EV fruits.
Supplemental Figure S6. Antisense downregulation of the D-limonene synthase gene causes the downregulation of genes required for monoterpenoid biosynthesis and the upregulation of genes related to different defense responses in plants.
Supplemental Figure S7. Quantitative real-time PCR analysis of selected genes (identified by microarray analyses) showing differential expression in two independent transgenic lines.
Supplemental Figure S8. JA measurement in the flavedo of AS and EV control plants 1 d and 5 d after Penicillium digitatum inoculation.
Supplemental Figure S9. Exogenous application of JA in regular mandarin fruits confers fungal resistance.
Supplemental Table S1. Accumulation of monoterpene hydrocarbons and monoterpene alcohols in AS and EV control fruits.
Supplemental Table S2. Differentially expressed genes in the intact mature flavedo of two independent transgenic AS cv Navelina sweet orange plants (versus EV plants).
Supplemental Table S3. Primers designed for quantitative real-time RT-PCR to evaluate the expression of genes selected from microarray analyses and from JA and isoprenoid pathways.
Glossary
- VOCs
volatile organic compounds
- GGDP
geranylgeranyl diphosphate
- SA
salicylic acid
- JA
jasmonic acid
- AS
antisense
- EV
empty vector
- cDNA
complementary DNA
- GO
Gene Ontology
- qRT
quantitative reverse transcription
- CDPK
calcium-dependent protein kinase
- MAPK
mitogen-activated protein kinase
- TF
transcription factor
- ABA
abscisic acid
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FW, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15: 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Jongsma MA, Bouwmeester HJ. (2005) Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci 10: 594–602 [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124: 783–801 [DOI] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16: 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbona V, Argamasilla R, Gómez-Cadenas A. (2010) Common and divergent physiological, hormonal and metabolic responses of Arabidopsis thaliana and Thellungiella halophila to water and salt stress. J Plant Physiol 167: 1342–1350 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Attaway JA, Pieringer AP, Barabas LJ. (1967) The origin of citrus flavor components. III. A study of the percentage variations in peel and leaf oil terpenes during one season. Phytochemistry 6: 25–32 [Google Scholar]
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ. (2006) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45: 982–993 [DOI] [PubMed] [Google Scholar]
- Ballester AR, Lafuente MT, Forment J, Gadea J, De Vos RC, Bovy AG, González-Candelas L. (2011) Transcriptomic profiling of citrus fruit peel tissues reveals fundamental effects of phenylpropanoids and ethylene on induced resistance. Mol Plant Pathol 12: 879–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester AR, Lafuente MT, González-Candelas L. (2006) Spatial study of antioxidant enzymes, peroxidase and phenylalanine ammonia-lyase in the citrus fruit-Penicillium digitatum interaction. Postharvest Biol Technol 39: 115–124 [Google Scholar]
- Bedon F, Bomal C, Caron S, Levasseur C, Boyle B, Mansfield SD, Schmidt A, Gershenzon J, Grima-Pettenati J, Séguin A, et al. (2010) Subgroup 4 R2R3-MYBs in conifer trees: gene family expansion and contribution to the isoprenoid- and flavonoid-oriented responses. J Exp Bot 61: 3847–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenbihl RP, Somssich IE. (2011) Transcriptional plant responses critical for resistance towards necrotrophic pathogens. Front Plant Sci 2: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Howe GA. (2008) New weapons and a rapid response against insect attack. Plant Physiol 146: 832–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassot C, Nawrath C, Métraux JP. (2007) Cuticular defects lead to full immunity to a major plant pathogen. Plant J 49: 972–980 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ, Shah J. (2012) An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J 71: 161–172 [DOI] [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, et al. (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BH (1976) Carotenoids. In TW Goodwin, ed, Chemistry and Biochemistry of Plant Pigments. Academic Press, New York, pp 38–165 [Google Scholar]
- Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. (2003) Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol 14: 169–176 [DOI] [PubMed] [Google Scholar]
- De Geyter N, Gholami A, Goormachtig S, Goossens A. (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17: 349–359 [DOI] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RM, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux JP, Van Loon LC, Dicke M, et al. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18: 923–937 [DOI] [PubMed] [Google Scholar]
- Droby S, Eick A, Macarisin D, Cohen L, Rafael G, Stange R, McColum G, Dudai N, Nasser A, Wisniewski M, et al. (2008) Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol Technol 49: 386–396 [Google Scholar]
- Droby S, Porat R, Cohen L, Weiss B, Shapiro B, Philosoph-Hadas S, Meir S. (1999) Suppressing green mold decay in grapefruit with postharvest jasmonate application. J Am Soc Hortic Sci 124: 184–188 [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I. (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25: 417–440 [Google Scholar]
- Dugo G, Di Giacomo A (2002) Citrus: The Genus Citrus Medicinal and Aromatic Plants: Industrial Profiles Series. Taylor & Francis Group, CRC Press, New York [Google Scholar]
- Durgbanshi A, Arbona V, Pozo Ó, Miersch O, Sancho JV, Gómez-Cadenas A. (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J Agric Food Chem 53: 8437–8442 [DOI] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. (2002) The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Turner JG. (2001) The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 13: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Reindl A, Reichler S, León P. (2001) 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276: 22901–22909 [DOI] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG. (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamini G, Cioni PL. (2010) Odour gradients and patterns in volatile emission of different plant parts and developing fruits of grapefruit (Citrus paradisi L.). Food Chem 120: 984–992 [Google Scholar]
- Flors V, Ton J, van Doorn R, Jakab G, García-Agustín P, Mauch-Mani B. (2008) Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. Plant J 54: 81–92 [DOI] [PubMed] [Google Scholar]
- Forment J, Gadea J, Huerta L, Abizanda L, Agusti J, Alamar S, Alos E, Andres F, Arribas R, Beltran JP, et al. (2005) Development of a citrus genome-wide EST collection and cDNA microarray as resources for genomic studies. Plant Mol Biol 57: 375–391 [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9: 436–442 [DOI] [PubMed] [Google Scholar]
- Gershenzon J. (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20: 1281–1328 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Goff SA, Klee HJ. (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311: 815–819 [DOI] [PubMed] [Google Scholar]
- Grotewold E. (2005) Plant metabolic diversity: a regulatory perspective. Trends Plant Sci 10: 57–62 [DOI] [PubMed] [Google Scholar]
- Guo YH, Yu YP, Wang D, Wu CA, Yang GD, Huang JG, Zheng CC. (2009) GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol 183: 62–75 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Baldwin I. (2004) Jasmonates and related compounds in plant-insect interactions. J Plant Growth Regul 23: 238–245 [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. (2008) Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiol 146: 974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HW, Kim KD, Hwang BK. (2005) Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta 221: 361–373 [DOI] [PubMed] [Google Scholar]
- Junker RR, Blüthgen N. (2010) Floral scents repel facultative flower visitors, but attract obligate ones. Ann Bot (Lond) 105: 777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. (2010) Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiol 152: 96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen TW, Luckerhoff LL, Dicke M, Bouwmeester HJ. (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Kavanagh JA, Wood RK. (1967) The role of wounds in the infection of oranges by Penicillium digitatum Sacc. Ann Appl Biol 60: 375–383 [Google Scholar]
- Kekelidze NA, Lomidze EP, Janikashvili MI. (1989) Analysis of terpene variation in leaves and fruits of Citrus unshiu Marc. during ontogenesis. Flavour Fragrance J 4: 37–41 [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. (2004) Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305: 665–668 [DOI] [PubMed] [Google Scholar]
- Kessler D, Gase K, Baldwin IT. (2008) Field experiments with transformed plants reveal the sense of floral scents. Science 321: 1200–1202 [DOI] [PubMed] [Google Scholar]
- Langenheim JH. (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20: 1223–1280 [DOI] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, Lee GI, Jayanty S, Sageman C, Vrebalov J, Giovannoni JJ, Yagi K, Kobayashi Y, et al. (2005) Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 17: 971–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT. (2010) Dispersers shape fruit diversity in Ficus (Moraceae). Proc Natl Acad Sci USA 107: 14668–14672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makandar R, Nalam VJ, Lee H, Trick HN, Dong Y, Shah J. (2012) Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact 25: 431–439 [DOI] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Godoy MA, Mauri N, Juarez J, Marques MC, Santiago J, Forment J, Gadea J. (2008) A genome-wide 20 K citrus microarray for gene expression analysis. BMC Genomics 9: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina I, Carbonell J, Pulido L, Madeira S, Goetz S, Conesa A, Tárraga J, Pascual-Montano A, Nogales-Cadenas R, Santoyo J, et al. (2010) Babelomics: an integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res 38: W210–W213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C. (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaise V, Roux M, Zipfel C. (2009) Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol 150: 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R. (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31: 1–12 [DOI] [PubMed] [Google Scholar]
- Pascual M, Mallent MD, Cuñat P. (1993) Estudio de los carotenoides de naranjas Navelina. Rev Esp Cien Tec Ali 33: 179–196 [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MA, Croteau RB. (1999) Resin-based defenses in conifers. Trends Plant Sci 4: 184–190 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411 [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Alférez F, Mallent MD, Zacarías L. (2003) Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J Exp Bot 54: 727–738 [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Marcos JF, Zacarías L. (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52: 6724–6731 [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Alquézar B, Peña L. (2013) Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol 197: 36–48 [DOI] [PubMed] [Google Scholar]
- Rodríguez A, San Andrés V, Cervera M, Redondo A, Alquézar B, Shimada T, Gadea J, Rodrigo M, Zacarías L, Palou L, et al. (2011a) The monoterpene limonene in orange peels attracts pests and microorganisms. Plant Signal Behav 6: 1820–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, San Andrés V, Cervera M, Redondo A, Alquézar B, Shimada T, Gadea J, Rodrigo MJ, Zacarías L, Palou L, et al. (2011b) Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol 156: 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Solano R, Sánchez-Serrano J. (2003) Interactions between signaling compounds involved in plant defense. J Plant Growth Regul 22: 82–98 [Google Scholar]
- Rouseff R, Raley L, Hofsommer H. (1996) Application of diode array detection with a C-30 reversed phase column for the separation and identification of saponified orange juice carotenoids. J Agric Food Chem 44: 2176–2181 [Google Scholar]
- Schaller F. (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52: 11–23 [PubMed] [Google Scholar]
- Schiestl FP. (2010) The evolution of floral scent and insect chemical communication. Ecol Lett 13: 643–656 [DOI] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103: 1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, et al. (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC. (2008) Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem 46: 941–950 [DOI] [PubMed] [Google Scholar]
- Skibbe M, Qu N, Galis I, Baldwin IT. (2008) Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 20: 1984–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Benitez A (1955) Chlorophylls. In K Paech, MV Tracey, eds, Modern Methods of Plant Analyses. Springer, Berlin, pp 142–196 [Google Scholar]
- Spiegel-Roy P, Goldschmidt EE (1996) The Biology of Citrus. Cambridge University Press, Cambridge, UK [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma S, Hecht U, Kippers A, Botella J, De Vries S, Somerville C. (1994) Tissue-specific expression of a gene encoding a cell wall-localized lipid transfer protein from Arabidopsis. Plant Physiol 105: 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF. (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Croteau R. (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
- Turner GW, Gershenzon J, Croteau RB. (2000) Development of peltate glandular trichomes of peppermint. Plant Physiol 124: 665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A. (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voo SS, Grimes HD, Lange BM. (2012) Assessing the biosynthetic capabilities of secretory glands in Citrus peel. Plant Physiol 159: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Guo Y, Wu C, Yang G, Li Y, Zheng C. (2008) Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genomics 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wu JH, Ng TB, Ye XY, Rao PF. (2004) A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 25: 1235–1242 [DOI] [PubMed] [Google Scholar]
- Wasternack C (2006) Oxylipins: biosynthesis, signal transduction and action. In P Hedden, S Thomas, eds, Plant Hormone Signaling. Annual Plant Reviews, Vol 24. Blackwell Publishing, Oxford, pp 185–228 [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Kombrink E. (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5: 63–77 [DOI] [PubMed] [Google Scholar]
- Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol 24: 1441–1447 [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Yang DH, Hettenhausen C, Baldwin IT, Wu J. (2012) Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol 159: 1591–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]