Abstract
We report two cases of lower-extremity furunculosis caused by Mycobacterium mageritense. Both patients were patrons of the same nail salon, where they received footbaths prior to pedicures. M. mageritense bacteria isolated from two whirlpool footbaths were determined to be closely related to the patient isolates by pulsed-field gel electrophoresis.
CASE REPORTS
Case 1.
A 43-year-old Caucasian woman presented to our clinic in December 2002 upon referral by an outside dermatologist, with a 3-month history of a culture-negative furunculosis on her right leg. She first recognized the lesion in September 2002 because it “felt like a spider bite.” Despite oral and topical antibiotic therapy and intralesional treatment with triamcinolone, the lesion continued to gradually enlarge. Biopsy samples collected by the referring dermatologist revealed inflamed granulation tissue and a neutrophilic abscess with no microorganisms visible in acid-fast bacillus (AFB)-, periodic acid-Schiff-, and Gram-stained tissue sections. The patient was otherwise in good health and denied any associated fever, headache, or upper respiratory tract symptoms.
On physical examination, this healthy-looking woman presented with a warm, fluctuant dull-red nodule on the pretibial area of her right leg; the lesion measured approximately 4 by 5 cm and had minimal draining exudate (Fig. 1). The overlying superficial ulcerations corresponded with prior biopsy sites. No other skin lesions were identified, and no lymphadenopathy was detected. A sample of the draining exudate was sent for bacterial, mycobacterial, and fungal cultures. A rapidly growing mycobacterium species isolated after 3 days of incubation on routine bacteriological media was later identified as Mycobacterium mageritense (7). Oral therapy with trimethoprim-sulfamethoxazole and levofloxacin was initiated, with gradual improvement of the lesion over the next 3 months.
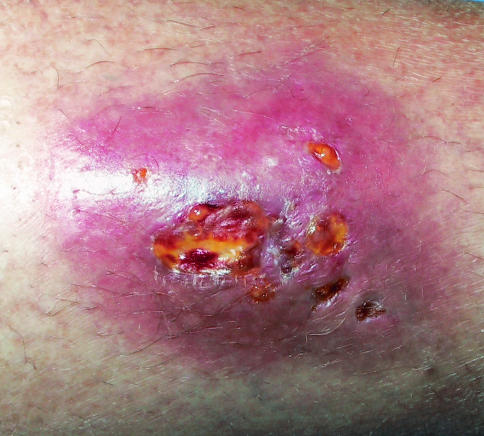
FIG. 1.
Nodule on the pretibial region of the right leg of patient 1. The 4- by 5-cm nodule was warm and fluctuant, with minimal draining serosanguinous exudate overlying superficial ulcerations.
Case 2.
A 56-year-old healthy Caucasian woman presented to our clinic in January 2003 upon referral by an outside dermatologist, with three ulcerating nodules in the pretibial area of the right leg; the first lesion had appeared in May 2002, the second had appeared in July 2002, and the third had appeared in October 2002. Previous cultures performed at other laboratories were negative. Like the first patient, this patient characterized the first lesion as feeling like an “insect bite.” Despite multiple topical and oral antibiotics as well as topical and intralesional corticosteroid therapy, the nodules continued to persist and enlarge. She denied having concurrent fever, fatigue, headache, or upper respiratory tract infections.
On physical examination, this healthy-appearing woman presented with three ulcerated, dull-red nodules configured in a sporotrichoid pattern on her right pretibial area, all measuring less than 5 cm. No lymphadenopathy was found. Punch biopsy samples were obtained and sent for microscopic evaluation and culture for bacteria, mycobacteria, and fungi. Histologic evaluation revealed a suppurative granulomatous dermatitis. No organisms were seen with special stains (AFB, periodic acid-Schiff, and Gram stains). The histologic features were similar to those seen during an earlier biopsy of the lesions performed at an outside institution. As with case 1, a rapidly growing mycobacterium was isolated from the biopsy specimen after 3 days of incubation and was identified as M. mageritense. The patient was treated with oral gatifloxacin with slow resolution of the lesions over a 2-month period.
The microbiological findings for both patients were similar. Direct stains for mycobacteria, fungi, and bacteria were negative, as were the mycobacterial and fungal cultures. After 3 days of incubation at 35°C, rough and smooth, nonpigmented bacterial colonies were isolated on sheep blood and chocolate agar plates. Gram stains prepared from the colonies showed beaded, gram-positive rods suggestive of a rapidly growing mycobacterium. This observation was confirmed with a Ziehl-Neelsen stain for acid-fast organisms. The rapidly growing mycobacteria grew on MacConkey agar without crystal violet and on Lowenstein-Jensen medium with 5% NaCl and were positive for arylsulfatase activity at 3 days, iron uptake, and nitrate reductase activity. Members of the Mycobacterium fortuitum group, which includes M. fortuitum, M. peregrinum, M. fortuitum third biovariant complex, and M. mageritense, all share this combination of phenotypic traits (15, 16).
PCR restriction enzyme analysis (PRA) of a 439-bp segment of the 65-kDa heat shock protein (hsp) gene (12) and high-performance liquid chromatography (HPLC) of mycolic acid esters (5) identified the isolates as M. mageritense (16). Broth microdilution antimicrobial susceptibility testing performed with cation-supplemented Mueller-Hinton broth showed that both patient isolates were susceptible to amikacin, imipenem, sulfamethoxazole, fluoroquinolones, and linezolid and resistant to clarithromycin (10). The isolate from case 1 was intermediate to cefoxitin, and the isolate from case 2 was susceptible to cefoxitin. This antimicrobial susceptibility profile is unique to isolates of M. mageritense (16).
Both of the patients were subsequently found to have received pedicures from the same nail salon on multiple occasions. Likewise, whirlpool footbaths had been given to both women as part of their pedicures at the salon. In addition, both women had shaved their legs with a razor prior to receiving their pedicures.
Based on the link between the two patients and the potential for a larger outbreak, the salon was visited on 27 February 2003 with inspectors from the Georgia State Board of Cosmetology to obtain environmental samples and to assess cleaning and disinfection procedures. The salon had 23 permanent pedicure stations with whirlpool footbaths. The water levels in the footbaths reached the midcalf region of the clients' legs.
We obtained multiple environmental samples from the salon for mycobacterial culture. Of the 23 whirlpool footbaths, 7 were randomly selected for sample collection. Using cotton-tipped swabs, we cultured behind the inlet suction screen of each of the seven basins, the inlet suction screen itself and the water jet in six basins, the drain in five basins, the overflow drain in two basins, the sprayer in one basin, the faucet and drain of the hand-washing sink, and the hot water heater drain. In addition, samples of standing water from a whirlpool footbath, tap water from the hand-washing sink, massage oils, lotions, cuticle oil, and cooling gel were collected.
Swab samples from the whirlpool footbaths were placed in tubes containing approximately 2 ml of Middlebrook 7H9 broth and decontaminated with 0.005% cetylpyridinium chloride. One milliliter of the broth specimen was filtered through a 0.45-μm-pore-size filter and washed twice with 100 ml of 0.00425% monopotassium phosphate buffer. The filter was placed on Middlebrook 7H10 agar with oleic acid-albumin-dextrose-catalase supplement and incubated at 35°C. Tap water samples were divided into three equal aliquots of approximately 300 ml. Two aliquots were decontaminated with either 0.005% cetylpyridinium chloride or 1% formaldehyde, and the third aliquot was not treated (6, 8). Each aliquot was filtered, and the filters were cultured as described above. Samples of commercial products used at the nail salon were inoculated directly on Middlebrook 7H10 agar with oleic acid-albumin-dextrose-catalase and incubated at 35°C. AFB colonies that grew on Middlebrook 7H10 agar were subcultured onto Lowenstein-Jensen agar for identification and further characterization.
While we did not find large amounts of hair or other debris behind the inlet suction screens of the footbaths, the cotton tips of the swabs were visibly soiled after swabbing every location in the footbaths. Cultures from three of the seven footbaths yielded M. mageritense, and cultures from two of the footbaths and the hand-washing sink drain yielded mycobacteria in the M. goodii-M. smegmatis group (3). M. mucogenicum was isolated from tap water from the hand-washing sink. All cultures of oils, lotions, and gels were negative for mycobacteria.
Patient and environmental isolates of M. mageritense were subtyped by pulsed-field gel electrophoresis (PFGE) (9) and repetitive-sequence PCR (rep-PCR) (14). Restriction of genomic DNA was performed with 40 U of XbaI (Invitrogen, Carlsbad, Calif.), and the resulting fragments were separated with a CHEF-DR system (Bio-Rad, Richmond, Calif.) at 14°C with pulse times ramped from 5 to 20 s at 200 V. PFGE patterns were interpreted by using criteria described by Tenover et al. (13).
Isolates for rep-PCR subtyping were subcultured on Wallenstein medium at 37°C, and the DNA was extracted by using a 1-μl loop of culture and an UltraClean Microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, Calif.) in accordance with the manufacturer's instructions and extending the 10-min microbead vortex step to 30 min. Genomic integrity was checked by agarose gel electrophoresis, and sample DNA was diluted to approximately 25 ng/μl based on a comparison of band intensities to that of a 25-ng standard. The extracted DNA (50 ng) was amplified by use of a DiversiLab DNA fingerprinting kit and Rep1R-I (Bacterial BarCodes, Houston, Tex.). Thermal cycling parameters were as follows: initial denaturation at 94°C for 2 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 70°C for 90 s; and a final extension at 70°C for 3 min. The amplicon separation, detection, and analysis were performed by use of the DiversiLab system. Separation and detection were performed with a Caliper Technologies Analyzer 1000. The resulting electropherograms were analyzed by use of Pearson's correlation coefficient and a distance tree constructed by clustering with the unweighted pair group method with arithmetic mean (http//www.icp.ucl.ac.be/∼opperd/private/upgma.html).
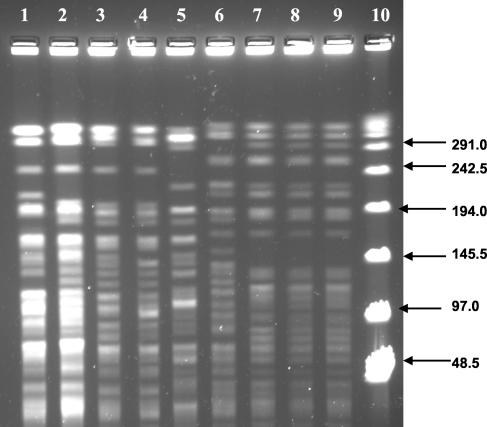
The PFGE patterns for the patient and environmental isolates of M. mageritense are shown in Fig. 2. The isolates from the patients and from footbaths 2 and 4 (Fig. 2, lanes 1 to 4) appeared to be closely related. Other environmental isolates from footbaths 2 and 4 appeared to represent another closely related clone (lanes 6 to 9). The remaining environmental isolate from footbath 22 (lane 5) had a unique PFGE pattern that was consistent with a third clone. The rep-PCR patterns generated were unique for each isolate; however, the three clones identified by PFGE formed three distinct clusters in the dendrogram based on the percent similarity of the rep-PCR electropherograms (data not shown).
FIG. 2.
PFGE analysis (XbaI) of M. mageritense isolates from patients and whirlpool footbaths as indicated for the respective lanes. Lane 1, case 2; lane 2, case 1; lane 3, footbath 2 (jet); lane 4, footbath 4 (behind inlet suction screen); lane 5, footbath 22 (inlet suction screen); lane 6, footbath 2 (inlet suction screen); lane 7, footbath 4 (jet); lane 8, footbath 2 (behind inlet suction screen); lane 9, footbath 2 (overflow drain); lane 10, 48.5-kb λ ladder.
Public health officials contacted clinical laboratories, dermatologists, and infectious disease specialists in the metropolitan Atlanta, Ga., area (Cobb, DeKalb, Fulton, and Gwinnett counties). Laboratories were asked to report any cultures positive for mycobacteria from lower-extremity lesions. Physicians were requested to report any patients with histories of persistent boils on the lower extremities who may have received pedicures at a nail salon and with onset of symptoms as early as June 2002.
Contacting laboratories and physicians identified no additional cases. One of the patients reported here reported another patient seen by her dermatologist for lesions on the lower legs with an onset in January 2003. Follow-up revealed that the patient had received a pedicure at a different salon, and laboratory testing was negative for mycobacteria. By the time this patient was interviewed, her lesions had completely resolved.
In May 2002, Winthrop et al. (17) reported an outbreak of lower-extremity mycobacterial furunculosis involving 110 otherwise healthy patrons of a nail salon in northern California. The outbreak was associated with pedicures that involved the use of contaminated whirlpool footbaths. M. fortuitum was identified as the etiologic agent, and isolates from the patients and the footbaths were indistinguishable by pulsed-field gel electrophoresis. Recently, Sniezek et al. described two patients with M. fortuitum infections and one patient with an M. abscessus infection of the lower extremity contracted after similar exposures to whirlpool footbaths at two different nail salons in southern California (11).
We report two cases of furunculosis caused by M. mageritense acquired from whirlpool footbaths in a nail salon in Georgia. Molecular subtyping of the patient and environmental isolates of M. mageritense corroborated the link between the patients and the nail salon. However, our case finding did not identify any additional cases of furunculosis associated with the salon. This is in sharp contrast with the outbreak of M. fortuitum furunculosis among patrons of a northern California nail salon (17).
Inherent differences in virulence between M. fortuitum and M. mageritense or mitigating environmental factors such as differences in the bacterial burdens of the footbaths may account for the small number of cases in our report. There may also have been factors that preferentially predisposed our two patients to infection, such as other skin lesions. However, the significant time lapse between the initial appearance of symptoms and evaluation at our institution makes this possibility difficult to evaluate. Although local physicians and laboratories were notified of a potential outbreak, additional cases that were not reported to public health authorities may have occurred. Also, as our two cases illustrate, a specific etiologic diagnosis depends heavily on the capability of the microbiology laboratory.
M. fortuitum, M. chelonae, M. smegmatis, and M. abscessus are among the more than 40 rapidly growing mycobacteria that are more commonly linked to human disease (4). Localized cutaneous infections manifesting as cellulitis, ulcerations, sinus tract infections, and abscesses are most common. Localized trauma to skin, medical procedures, surgery, and orthopedic implants increase the risk of infection with these mycobacteria. Both of our patients reported shaving their legs prior to their footbaths and pedicures, a previously identified risk factor in a nail salon outbreak (17).
M. mageritense is a recently described, nonpigmented rapidly growing mycobacterium with closest similarity to the M. fortuitum third biovariant complex (7). It was first isolated from sputum samples from five patients seen at two hospitals in Spain. None of the isolates were known to be clinically significant. Wallace et al. (16) described the first isolates of this mycobacterium from patients in the United States. The six U.S. isolates were recovered from a variety of sources, including the respiratory tract, wounds, and blood. Several of these isolates were associated with disease.
Our report further documents the pathogenic potential of M. mageritense and is the first to describe its isolation from an environmental source. M. mageritense, M.goodii or M. smegmatis, and M. mucogenicum were all isolated from various water and footbath samples; however, M. mageritense was the most common environmental isolate. Given that rapidly growing mycobacteria are ubiquitous in most municipal water supplies, it is likely that M. mageritense was introduced by the tap water into the footbaths and was able to thrive in the warm, nutrient-rich water. The water levels in the footbaths was above the sites of the lesions in both patients.
M. mageritense produces nonpigmented colonies in 2 to 5 days at temperatures ranging from 22 to 45°C. M. mageritense can grow on a variety of routine bacteriologic media and on specialized media developed for recovery and differentiation of mycobacteria, including Lowenstein-Jensen medium with 5% NaCl and MacConkey agar without crystal violet. These strains display positive tests for arylsulfatase (in 3 days), nitrate reductase, iron uptake, and urease. They can utilize a variety of different carbohydrates for growth, including d-mannitol, i-myo-inositol, l-rhamnose, d-trehalose, and sorbitol. Most of these phenotypic characteristics are shared with sorbitol-positive isolates of M. fortuitum third biovariant complex, and it is likely that at least some of the strains that have been assigned to this complex to date are actually M. mageritense. However, utilization of l-rhamnose distinguishes M. mageritense from sorbitol-positive M. fortuitum third biovariant complex. Definitive identification of an isolate as M. mageritense can be accomplished by 16S rRNA partial gene sequencing, PRA of the 65-kDa hsp gene, or HPLC of mycolic acid esters (16). The identifications of our patient isolates were confirmed by both PRA and HPLC.
It is interesting to note the seemingly paradoxical culture results in our laboratory. The mycobacterial cultures were negative, but the routine bacterial cultures revealed rapidly growing mycobacteria after 3 days of incubation. The portions of the specimens cultured for mycobacteria were first digested and decontaminated with N-acetyl-l-cysteine to prevent overgrowth by other microorganisms. M. mageritense may be more susceptible than other mycobacteria to the adverse effects of specimen decontamination. Although rapidly growing mycobacteria grow rapidly in relation to other mycobacteria, and will grow on routine bacteriological media, laboratories would need to hold routine bacterial cultures for a minimum of 3 days to ensure the recovery of these organisms.
The antimicrobial susceptibility patterns previously reported for 12 previously described isolates of M. mageritense are very similar. All have been susceptible to ciprofloxacin and sulfamethoxazole, susceptible or intermediate to amikacin and cefoxitin, and resistant to clarithromycin. This same pattern was found with our clinical isolates. Resistance to clarithromycin is seen in both M. mageritense and sorbitol-positive M. fortuitum third biovariant complex; however, the two groups of organisms can be distinguished by cefoxitin MICs. The cefoxitin MICs for all M. mageritense isolates are ≤32 μg/ml, while those for all sorbitol-positive isolates of M. fortuitum third biovariant complex are ≥64 μg/ml (16).
The optimal treatment for M. mageritense infections is not defined, but considering the antimicrobial susceptibility profile of this organism, regimens described for M. fortuitum complex infections hold the most promise. M. fortuitum complex infections usually respond to surgical excision of the infected area and 6 to 12 months of oral therapy with two drugs to which it is susceptible (1). Both of our patients responded to antimicrobial therapy within 2 to 3 months without surgical intervention.
Both of our patients described their initial lesions as insect bites. Consequently, the lesions were treated both as infected arthropod bites and as persistent arthropod reactions with antibiotics and corticosteroids, respectively. However, the lesions continued to slowly progress. Despite adequate sampling of the lesions by referring physicians, the cultures for both women performed at other laboratories were repeatedly negative. This points to the variable capabilities of laboratories to isolate M. mageritense and demonstrates that these infections may go unrecognized without appropriate laboratory support.
In the northern California outbreak, Winthrop et al. reported large amounts of hair and skin debris behind the inlet suction screens of the sampled footbaths (17). We did not observe this at the salon we investigated, and the State Board of Cosmetology inspectors reported a level of visual cleanliness comparable to or better than that at most nail salons. Nonetheless, rapidly growing mycobacteria were identified in several locations and may be prevalent in other salons. We provided written cleaning and disinfection instructions to the nail salon and followed up with telephone calls to reinforce the procedures. To prevent infections, state public health authorities should review and, if necessary, revise nail salon inspection procedures and cleaning and disinfection guidelines. In addition, clinicians should obtain a history of nail salon exposure from patients with furunculosis or other soft-tissue infections of the lower extremities and should notify public health authorities of such infections so that outbreaks may be identified and control measures may be initiated.
The nail industry has experienced unprecedented growth. An industry survey conducted in 2002 estimated the total number of nail salons in the United States to be 51,571 and the total number of licensed nail technicians to be 368,818 (2). According to the same survey, Americans spend an estimated 6.45 billion dollars on nail salon services. The present report is the third describing cases of mycobacterial furunculosis associated with receiving whirlpool footbaths at a nail salon. Considering the inadequacy of regulations concerning the cleaning and disinfection of these footbaths and the growth in the nail care industry, it is likely that the incidence of these infections may increase. These infections are difficult to diagnose without appropriate laboratory support and, consequently, may be underrecognized. Rapidly growing mycobacteria should be considered in the differential diagnosis of soft-tissue infections of the legs, particularly in patrons of nail salons.
Acknowledgments
We thank Barbara Brown-Elliott of the Mycobacterial/Nocardia Research Laboratory, University of Texas Health Center, Tyler, Tex., for performing the PRA of the 65-kDa hsp gene and the antimicrobial susceptibility testing of the patient isolates and Mimi Healy of Bacterial BarCodes, Inc., for performing the rep-PCR subtyping of the patient and environmental isolates. We also thank Matthew Arduino and Terri Forster of the Centers for Disease Control and Prevention for their assistance with the environmental cultures.
REFERENCES
- 1.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Part 2. Am. J. Respir. Crit. Care Med. 156:1S-25S. [DOI] [PubMed]
- 2.Anonymous. 2001. Fact book—2000-2001. Nails Mag. 18:33-62. [Google Scholar]
- 3.Brown, B. A., B. Springer, V. A. Steingrube, R. W. Wilson, G. E. Pfyffer, M. J. Garcia, M. C. Menendez, B. Rodriguez-Salgado, K. C. Jost, Jr., S. H. Chiu, G. O. Onyi, E. C. Bottger, and R. J. Wallace, Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Bacteriol. 49:1493-1511. [DOI] [PubMed] [Google Scholar]
- 4.Brown, B. A., and R. J. Wallace, Jr. 2000. Infection due to nontuberculous mycobacteria, p. 2630-2636. In G. L. Mandell, J. E. Bennett, and R. Doland (ed.), Mandell, Douglas and Bennett's principles and practice of infectious diseases, 5th ed., vol. 2. Churchill Livingston, Philadelphia, Pa. [Google Scholar]
- 5.Butler, W. R., K. C. Jost, Jr., and J. O. Kilburn. 1991. Identification of mycobacteria by high-performance liquid chromatography. J. Clin. Microbiol. 29:2468-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson, L. A., L. B. Cusick, L. A. Bland, and M. S. Favero. 1988. Efficacy of chemical dosing methods for isolating nontuberculous mycobacteria from water supplies of dialysis centers. Appl. Environ. Microbiol. 54:1756-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domenech, P., M. S. Jimenez, M. C. Menendez, T. J. Bull, S. Samper, A. Manrique, and M. J. Garcia. 1997. Mycobacterium mageritense sp. nov. Int. J. Syst. Bacteriol. 47:535-540. [DOI] [PubMed] [Google Scholar]
- 8.Falkinham, J. O., III, C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hector, J. S., Y. Pang, G. H. Mazurek, Y. Zhang, B. A. Brown, and R. J. Wallace, Jr. 1992. Large restriction fragment patterns of genomic Mycobacterium fortuitum DNA as strain-specific markers and their use in epidemiologic investigation of four nosocomial outbreaks. J. Clin. Microbiol. 30:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCLS. 2003. Susceptibility testing of mycobacteria, nocardia and other aerobic actinomycetes. Approved standard. NCCLS document M24-A. NCCLS, Wayne, Pa. [PubMed]
- 11.Sniezek, P. J., B. S. Graham, H. Byers Busch, E. R. Lederman, M. L. Lim, K. Poggemyer, A. Kao, M. Mizrahi, G. Washabaugh, M. Yakrus, and K. L. Winthrop. 2003. Rapidly growing mycobacterial infections after pedicures. Arch. Dermatol. 139:629-634. [DOI] [PubMed] [Google Scholar]
- 12.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent, V., B. A. Brown-Elliot, K. C. Jost, Jr., and R. J. Wallace, Jr. 2003. Mycobacterium: phenotypic and genotypic identification, p. 560-584. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 16.Wallace R. J., Jr., B. A. Brown-Elliott, L. Hall, G. Roberts, R. W. Wilson, L. B. Mann, C. J. Crist, S. H. Chiu, R. Dunlap, M. J. Garcia, J. T. Bagwell, and K. C. Jost, Jr. 2002. Clinical and laboratory features of Mycobacterium mageritense. J. Clin. Microbiol. 40:2930-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winthrop, K. L., M. Abrams, M. Yakrus, I. Schwartz, J. Ely, D. Gillies, and D. J. Vugia. 2002. An outbreak of mycobacterial furunculosis associated with footbaths at a nail salon. N. Engl. J. Med. 346:1366-1371. [DOI] [PubMed] [Google Scholar]