Figure 1.
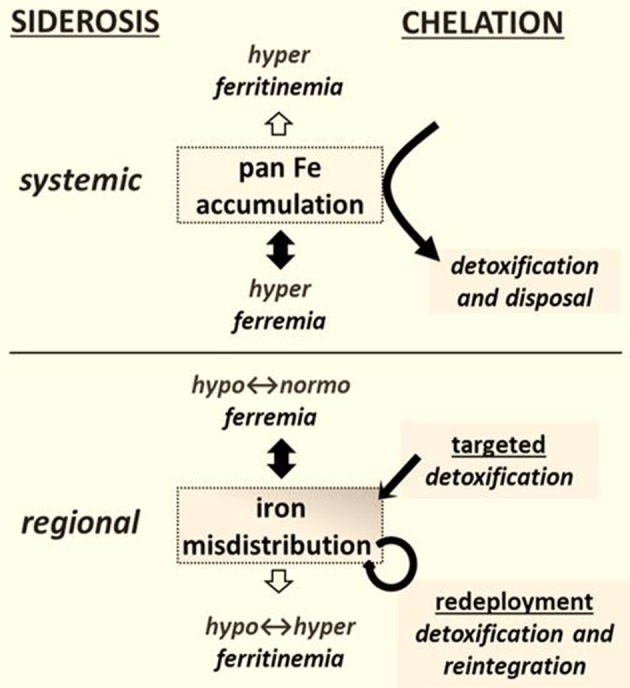
Chelation modalities as treatments for siderosis. Systemic siderosis (e.g., hemosiderosis) is characterized by elevated plasma iron levels (hyperferremia) that leads to organ iron accumulation and is generally characterized by elevated plasma ferritin (i.e., hyperferritinemia) that other than in a context of inflammation reflects iron stores. Chelation is designed to detoxify organs from surplus iron and dispose the latter via biliary or urinary secretion. Regional siderosis covers a wide spectrum of inherited disorders (e.g., Friedreich ataxia, sideroblastic anemias, iron-refractory-iron-deficiency anemia-IRIDA), and acquired disorders (e.g., anemia of chronic disease-ACD or cancer) that are characterized by a maldistribution of iron within cells of particular organs (e.g., cardiomyocytes or neurons or blast cells) or at the level of the organism (e.g., liver and spleen versus plasma). The plasma iron levels can span from subnormal (as in functional iron deficient anemias) to supranormal (as in sideroblastic anemias) and likewise those of plasma (or serum) ferritin levels. Chelation is designed here not merely to detoxify a siderotic region but where applicable render the chelated iron available for reuse. The new chelation modalities comprise (i) targeted detoxification, whereby a prochelator is activated at the target site by specific resident activators, as found in some brain areas (Sohn et al., 2008; Zheng et al., 2009) and (ii) iron redeployment, whereby a chelator that detoxifies cells from surplus iron and/or also scavenges essential iron reintegrates the metal into the erythron or specific tissues (Breuer and Cabantchik, 2009; Sohn et al., 2011).
